Wavelinqв ў Endoavf System Bd Canada
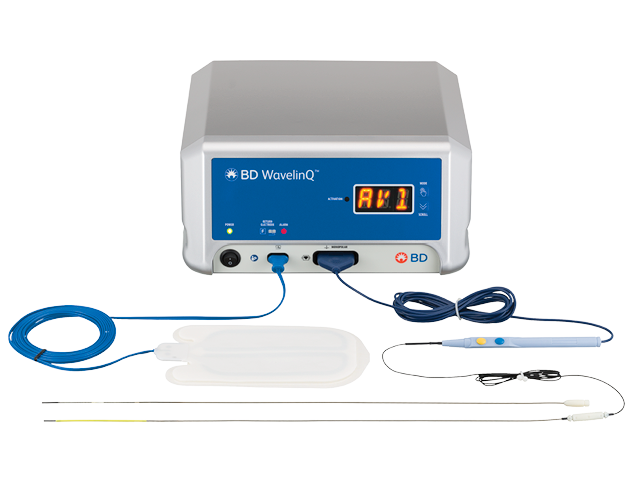
Wavelinqв ў Endoavf System Bd Wavelinq™ endoavf system for endovascular av fistula creation. the wavelinq™ endoavf system venous catheter is a flexible 4f magnetic catheter that contains a radiofrequency (rf) electrode for the delivery of rf energy, a yellow valve crosser on the distal end, and a cable and plug. The wavelinq™ endoavf system is designed to give you a versatile endovascular av fistula creation alternative to open surgery. while av fistulas are the preferred access option for eskd patients, surgical creation has challenges. endoavf offers patients potential benefits compared to current surgical creation options.

Clinical Utility Of The Wavelinqв ў Endoavf System Endovascular Today The wavelinq™ endoavf system is indicated for the creation of an arteriovenous fistula (avf) using concomitant ulnar artery and ulnar vein or concomitant radial artery and radial vein in patients with minimum artery and vein diameters of 2.0 mm at the fistula creation site who have chronic kidney disease and need haemodialysis. Why endoavf? the wavelinq™ endoavf system: minimizes arm disfigurement ; provides additional anatomical av fistula locations; allows for multiple venous procedural approaches 1; our product the wavelinq™ endoavf system consists of a 4f venous and a 4f arterial magnetic catheter. Wavelinq™ endoavf system for endovascular av fistula creation. the wavelinq™ endoavf system venous catheter is a flexible 4f magnetic catheter that contains a radiofrequency (rf) electrode for the delivery of rf energy, a yellow valve crosser on the distal end, and a cable and plug. Overview. the wavelinq™ endoavf system is indicated for the creation of an arteriovenous fistula (avf) using concomitant ulnar artery and ulnar vein or concomitant radial artery and radial vein in patients with minimum artery and vein diameters of 2.0 mm at the fistula creation site who have chronic kidney disease and need hemodialysis.

Comments are closed.