Using Lewis Structures And Formal Charge Which Of The Following Ions
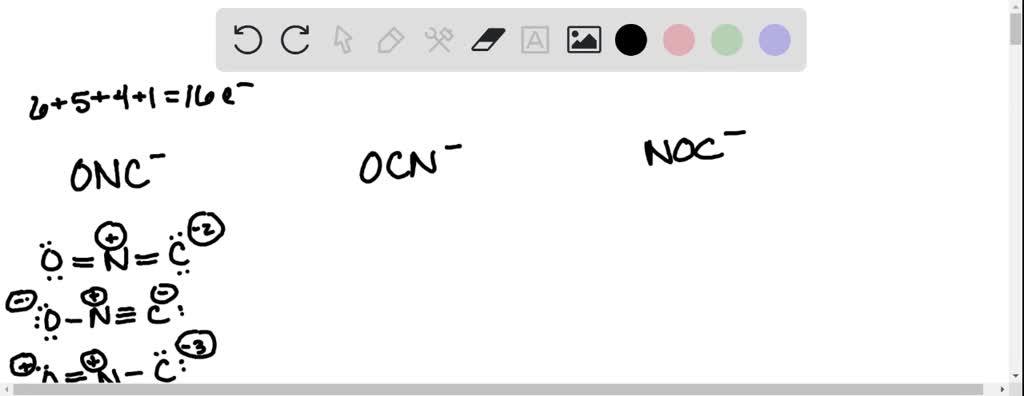
Solved Using Lewis Structures And Formal Charge Which Of The 57) using lewis structures and formal charge, which of the following ions is most stable? ocn⁻ onc⁻ noc⁻ a) ocn⁻ b) onc⁻ c) noc⁻ d) none of these ions are stable according to lewis theory. e) all of these compounds are equally stable according to lewis theory. Using lewis structures and formal charge, which of the following ions is most stable? ocn onc noc * onc * noc false * ocn * none of these ions are stable according to lewis theory. * all of these compounds are equally stable according to lewis theory.

Draw Lewis Structures And Show All Formal Charges For These Ions A To find formal charges in a lewis structure, for each atom, you should count how many electrons it "owns". count all of its lone pair electrons, and half of its bonding electrons. the difference between the atom's number of valence electrons and the number it owns is the formal charge. for example, in nh 3, n has 1 lone pair (2 electrons) and 3. The 2 charge means that there are 2 extra electrons. total: 4 (3 × 6) 2 = 24 electrons. the final answer must have this number of electrons‼! step 2) attach the atoms to each other using single bonds (“draw the skeleton structure”) step 3) add electrons to all outer atoms (except h) to complete their octets. Calculating formal charge from lewis structures assign formal charges to each atom in the interhalogen molecule brcl 3. solution. step 1. assign one of the electrons in each br–cl bond to the br atom and one to the cl atom in that bond: step 2. assign the lone pairs to their atom. now each cl atom has seven electrons and the br atom has seven. Net charges are shown outside the brackets. solutions to exercises. 1.6: lewis structures and formal charges (review) is shared under a license and was authored, remixed, and or curated by libretexts. lewis structures show us how atoms come together to create compounds and ions according to the octet rule.

Comments are closed.