Structure Of Haemoglobin 1980
/revision/latest/scale-to-width-down/330?cb=20191110171724)
The Structure And Function Of Haemoglobin Educational Film Wiki Fandom Structure and function of hemoglobin. the primary function of hb is to transport oxygen (o 2) from the lung to tissues, binding and releasing o 2 in a cooperative manner, as demonstrated by the oxygen equilibrium curve (oec), which represents o 2 saturation of hb (so 2) at varying partial pressures of o 2 (po 2) (fig. 14.1). The hemoglobin molecule (or "hb") is a tetramer of two α and two β chains, of 141 and 146 residues in human. they are different but homologous, with a "globin fold" structure similar to myoglobin. here we see a single of hemoglobin, starting with an overview of the subunit.
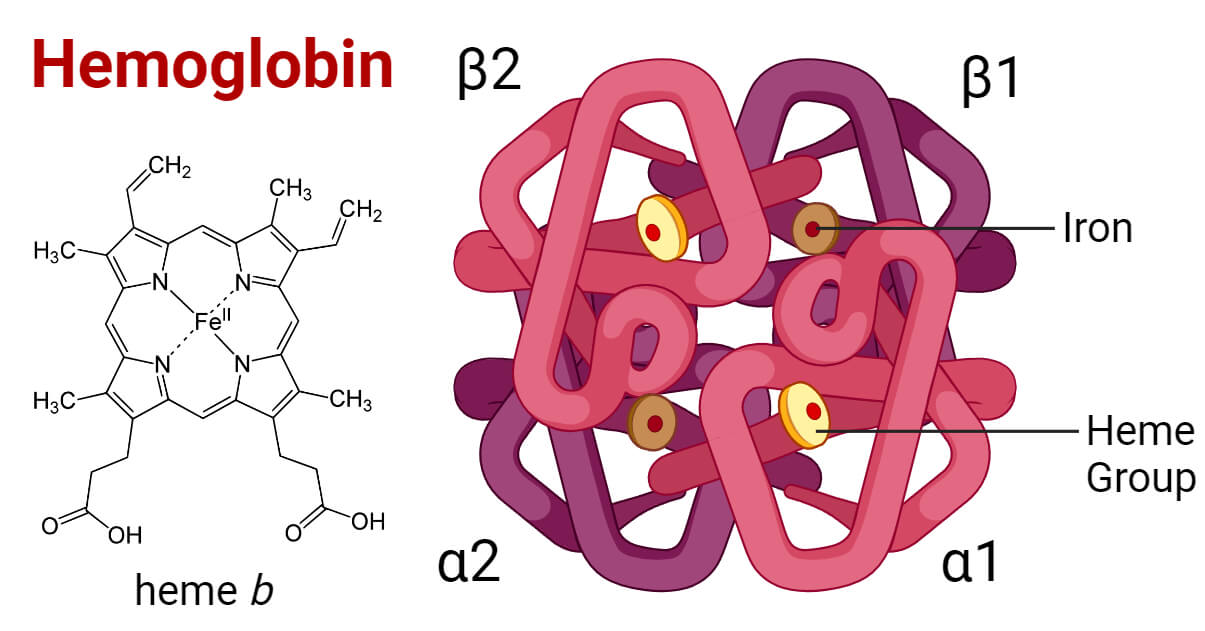
Hemoglobin Structure Types Functions Diseases Hemoglobin is a two way respiratory carrier, transporting oxygen from the lungs to the tissues and facilitating the return transport of carbon dioxide. in the arterial circulation, hemoglobin has a high affinity for oxygen and a low affinity for carbon dioxide, organic phosphates, and hydrogen and chloride ions. Hemoglobin (haemoglobin, [a] hb or hgb) is a protein containing iron that facilitates the transport of oxygen in red blood cells. almost all vertebrates contain hemoglobin, [ 3 ] with the sole exception of the fish family channichthyidae . [ 4 ]. Energy, plants and man the structure and function ofhaemoglobin dear sir, ihave read, with interest, w k pickeriug's criticism of my text 'energy a computer generated 3 d anaglyph film from theuniversity of london in association wi sfw h utrecht. 16 ram film, 3 d(red green) sp ctacles, plants and man' (b ochem c.a f ,d (1980) (1) 32). Haemoglobin (hb) is widely known as the iron containing protein in blood that is essential for o 2 transport in mammals. less widely recognised is that erythrocyte hb belongs to a large family of hb proteins with members distributed across all three domains of life—bacteria, archaea and eukaryotes. this review, aimed chiefly at researchers.
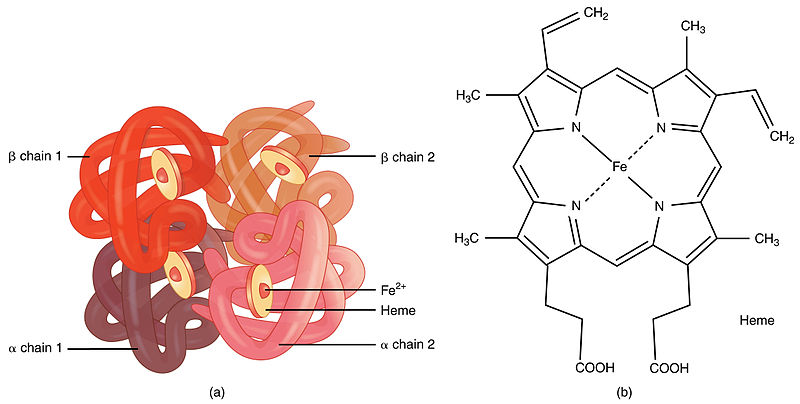
Hemoglobin Facts Structure Summary Synthesis Function Energy, plants and man the structure and function ofhaemoglobin dear sir, ihave read, with interest, w k pickeriug's criticism of my text 'energy a computer generated 3 d anaglyph film from theuniversity of london in association wi sfw h utrecht. 16 ram film, 3 d(red green) sp ctacles, plants and man' (b ochem c.a f ,d (1980) (1) 32). Haemoglobin (hb) is widely known as the iron containing protein in blood that is essential for o 2 transport in mammals. less widely recognised is that erythrocyte hb belongs to a large family of hb proteins with members distributed across all three domains of life—bacteria, archaea and eukaryotes. this review, aimed chiefly at researchers. Hemoglobin e. hemoglobin, iron containing protein in the blood of many animals—in the red blood cells (erythrocytes) of vertebrates —that transports oxygen to the tissues. hemoglobin forms an unstable reversible bond with oxygen. in the oxygenated state, it is called oxyhemoglobin and is bright red; in the reduced state, it is purplish blue. Abstract. human hemoglobin (hb) is the erythrocyte hemeprotein resulting from the combination of one pair of α like (α or ζ) chains and another pair of β like (β, δ, γ or ε) chains. each of these chains is associated with a heme prosthetic group, a tetrapyrrole ring (protoporphyrin ix) containing a central ferrous atom (fe 2 ), which.
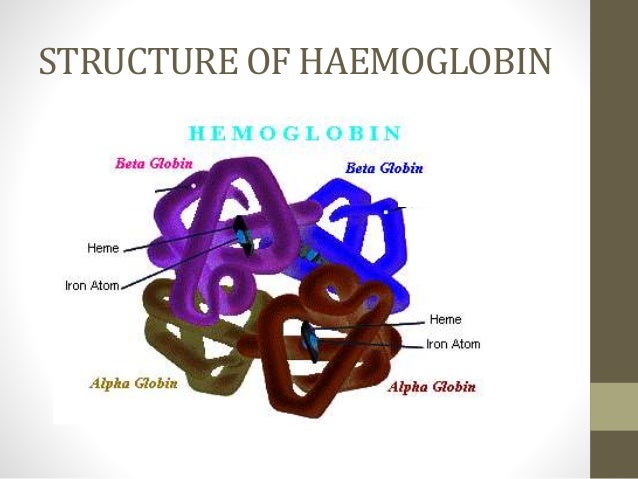
Structure Of Haemoglobin Hemoglobin e. hemoglobin, iron containing protein in the blood of many animals—in the red blood cells (erythrocytes) of vertebrates —that transports oxygen to the tissues. hemoglobin forms an unstable reversible bond with oxygen. in the oxygenated state, it is called oxyhemoglobin and is bright red; in the reduced state, it is purplish blue. Abstract. human hemoglobin (hb) is the erythrocyte hemeprotein resulting from the combination of one pair of α like (α or ζ) chains and another pair of β like (β, δ, γ or ε) chains. each of these chains is associated with a heme prosthetic group, a tetrapyrrole ring (protoporphyrin ix) containing a central ferrous atom (fe 2 ), which.

Comments are closed.