Solved Using Formal Charges Determine Which Lewis Structure Is The
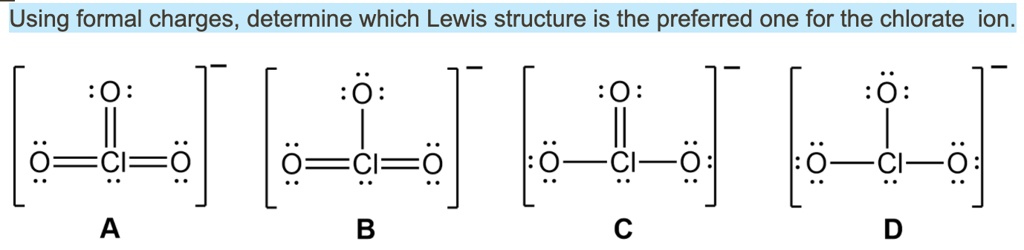
Solved Using Formal Charges Determine Which Lewis Structure Is The Answer to using formal charges, determine which lewis structure there are 2 steps to solve this one. using formal charges, determine which lewis structure is. To find formal charges in a lewis structure, for each atom, you should count how many electrons it "owns". count all of its lone pair electrons, and half of its bonding electrons. the difference between the atom's number of valence electrons and the number it owns is the formal charge. for example, in nh 3, n has 1 lone pair (2 electrons) and 3.
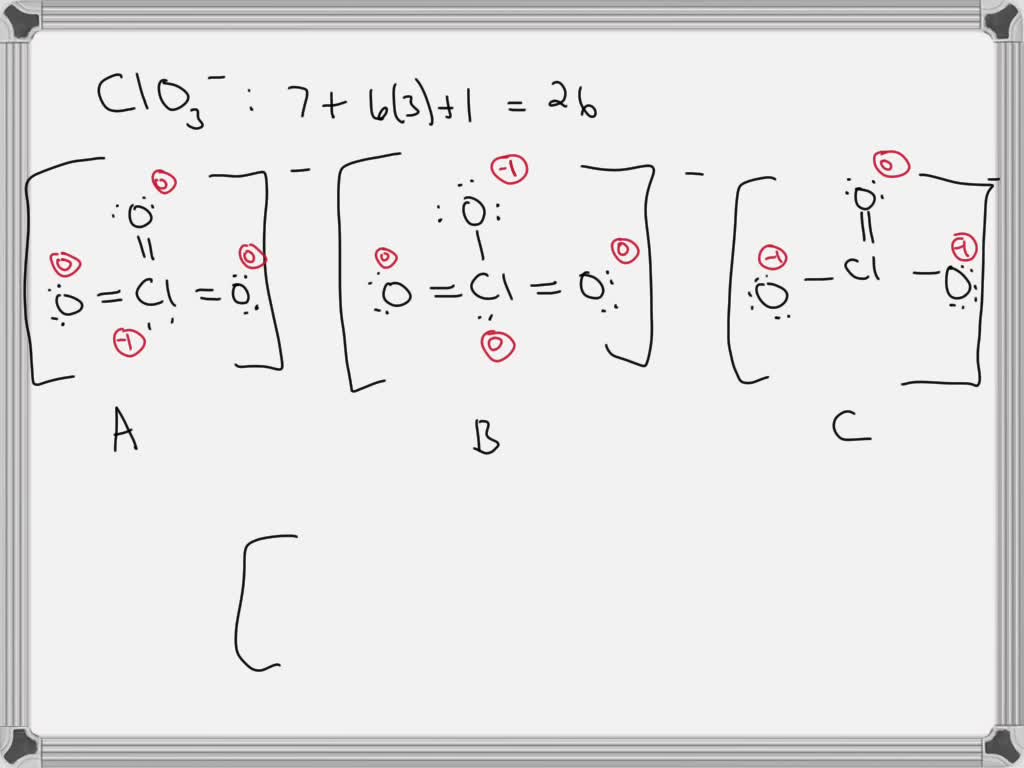
Solved Using Formal Charges Determine Which Lewis Structure Is The Question: using formal charges, determine which lewis structure is the preferred one for the suition 😮 :: 0: 2 a b c d 05 o a. here’s the best way to solve it. calculate the total number of valence electrons for the sulfite ion s o 3 2 −. the correct …. Formal charge. 10.7: formal charges is shared under a not declared license and was authored, remixed, and or curated by libretexts. in a lewis structure, formal charges can be assigned to each atom by treating each bond as if one half of the electrons are assigned to each atom. these hypothetical formal charges are a guide to …. The lewis electron structure for the nh 4 ion is as follows: the nitrogen atom shares four bonding pairs of electrons, and a neutral nitrogen atom has five valence electrons. using equation 4.4.1, the formal charge on the nitrogen atom is therefore. formalcharge(n) = 5 −(0 8 2) = 0. The most correct lewis structure will be the structure where the formal charges are evenly distributed throughout the molecule. the sum of all the formal charges should equal the total charge of the molecule. formal charge is the difference between the number of valence electrons of each atom and the number of electrons the atom is associated.
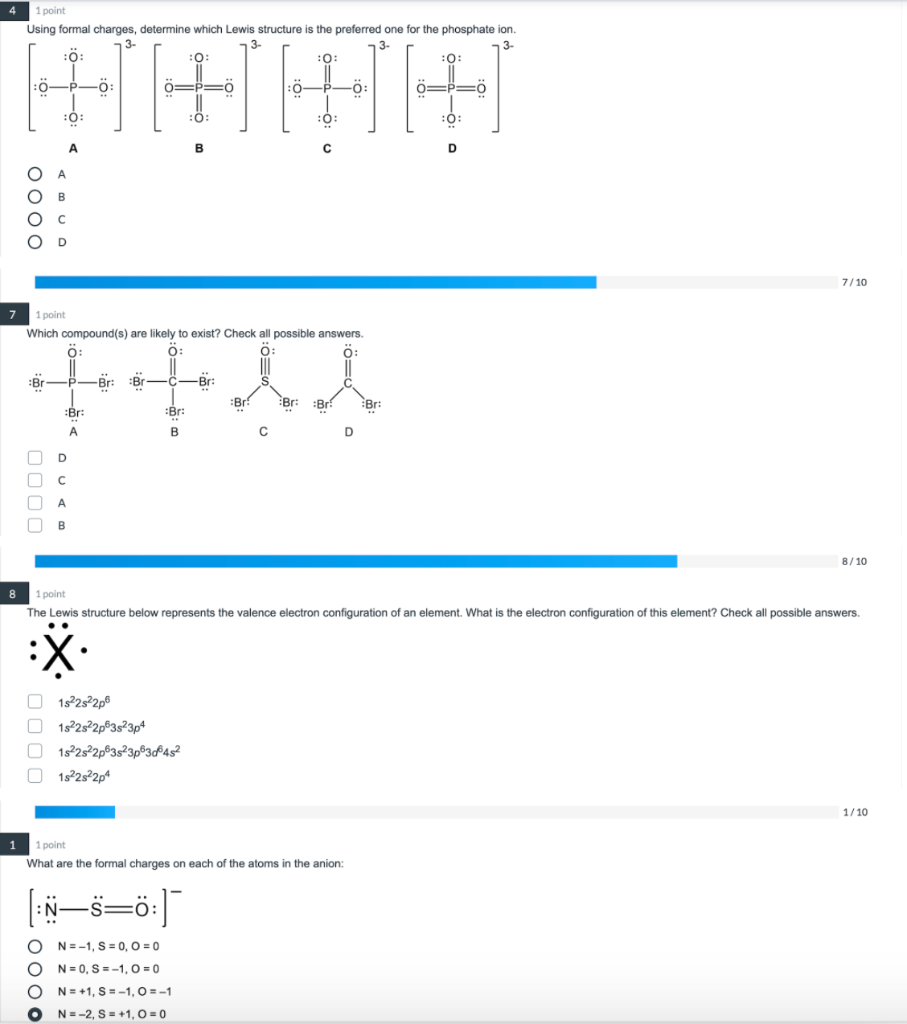
Solved 4 1 Point Using Formal Charges Determine Which Lewis Cheg The lewis electron structure for the nh 4 ion is as follows: the nitrogen atom shares four bonding pairs of electrons, and a neutral nitrogen atom has five valence electrons. using equation 4.4.1, the formal charge on the nitrogen atom is therefore. formalcharge(n) = 5 −(0 8 2) = 0. The most correct lewis structure will be the structure where the formal charges are evenly distributed throughout the molecule. the sum of all the formal charges should equal the total charge of the molecule. formal charge is the difference between the number of valence electrons of each atom and the number of electrons the atom is associated. Calculating formal charge from lewis structures assign formal charges to each atom in the interhalogen molecule brcl 3. solution. step 1. assign one of the electrons in each br–cl bond to the br atom and one to the cl atom in that bond: step 2. assign the lone pairs to their atom. now each cl atom has seven electrons and the br atom has seven. How to calculate formal charge. once we add all the formal charges for the atoms in the lewis structure, we should get a value equal to the actual charge of the molecule or ion. if it is a neutral molecule, then the sum of all the formal charges must equal zero. if it is a molecular ion, then the sum of all the formal charges must equal the.

Solved 5 Point Using Formal Charges Determine Which Lewis Chegg Calculating formal charge from lewis structures assign formal charges to each atom in the interhalogen molecule brcl 3. solution. step 1. assign one of the electrons in each br–cl bond to the br atom and one to the cl atom in that bond: step 2. assign the lone pairs to their atom. now each cl atom has seven electrons and the br atom has seven. How to calculate formal charge. once we add all the formal charges for the atoms in the lewis structure, we should get a value equal to the actual charge of the molecule or ion. if it is a neutral molecule, then the sum of all the formal charges must equal zero. if it is a molecular ion, then the sum of all the formal charges must equal the.
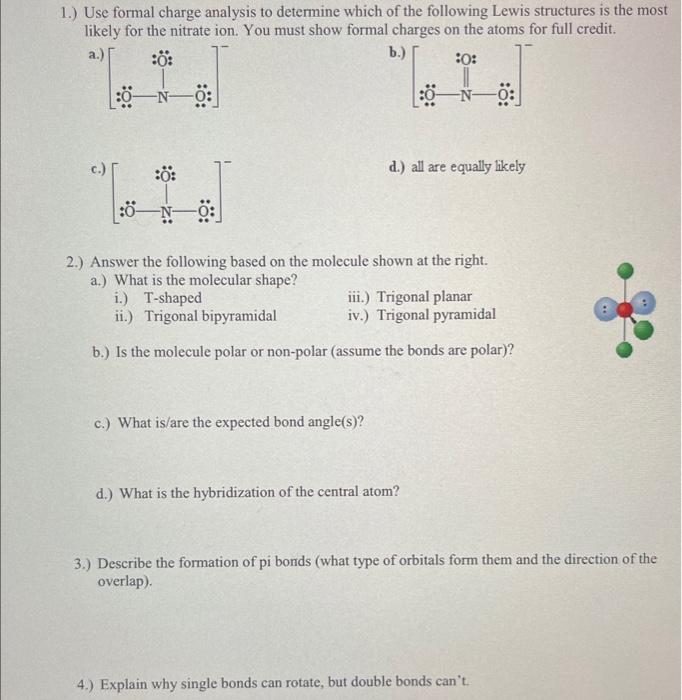
Solved 1 Use Formal Charge Analysis To Determine Which

Comments are closed.