Solved A Student Proposes The Following Lewis Structure For The
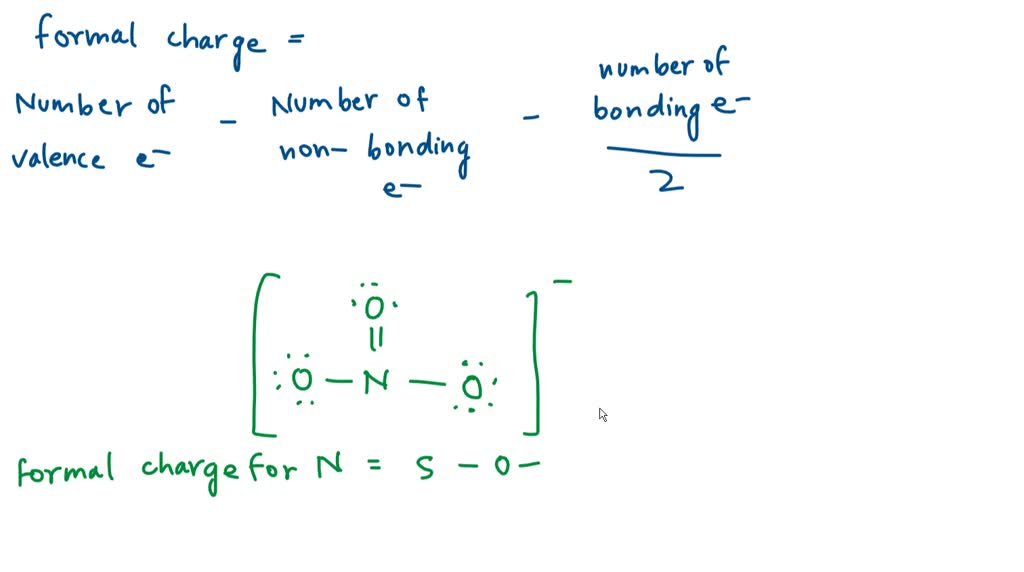
Solved A Student Proposes The Following Lewis Structure For The Our expert help has broken down your problem into an easy to learn solution you can count on. question: a student proposes the following lewis structure for the phosgene cocl2 molecule. assign a formal charge to each atom in the student's lewis structure. a student proposes the following lewis structure for the phosgene cocl2 molecule. Question: a student proposes the following lewis structure for the peroxide (o2 ) ion. 2 : o o: assign a formal charge to each atom in the student's lewis structure. atom formal charge left o right o. there are 3 steps to solve this one.

Solved A Student Proposes The Following Lewis Structure For The "a student proposes the following lewis structure for the carbonate (co; ion assign formal charge to each atom in the student's lewis structure atom formal charge central 0 right 0 bottom 0" submitted by laura s. dec. 09, 2021 08:29 p.m. Step 1 2 first, we need to determine the number of valence electrons for the isocyanate ion. isocyanate ion has the formula nco , which means it has one nitrogen atom, one carbon atom, and one oxygen atom, plus one extra electron to give it a negative charge. A student proposes the following lewis structure for the carbon dioxide (co2) molecule. assign a formal charge to each atom in the student's lewis structure atom formal charge x g 1eft o c right o instant answer. Click here 👆 to get an answer to your question ️ a student proposes the following lewis structure for the isocyanate (nco^ ) ion. [c=0=n ·s end(bmatrix)^.
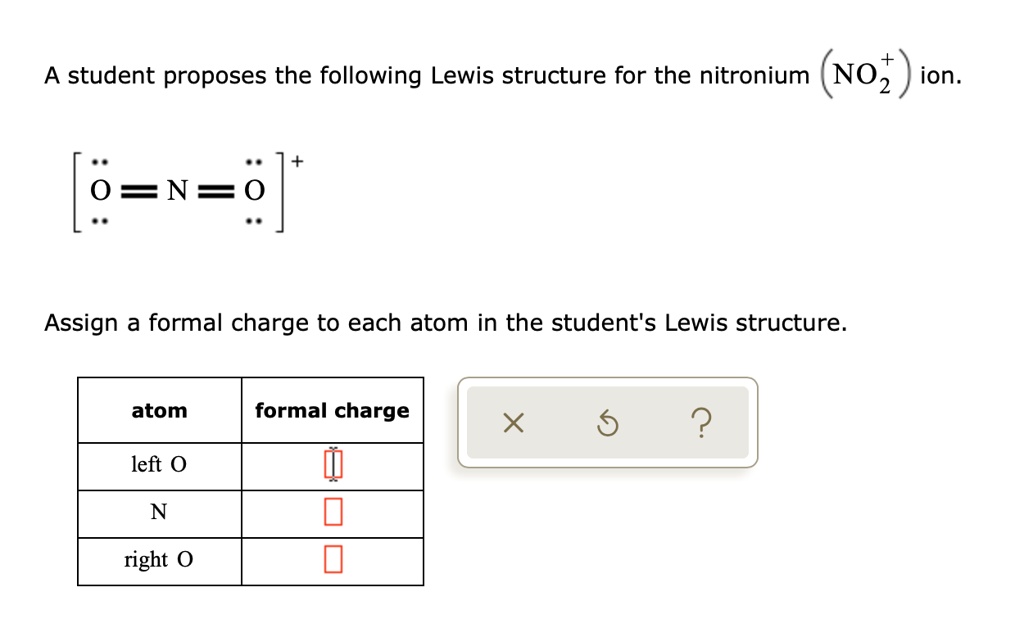
Solved A Student Proposes The Following Lewis Structure For The A student proposes the following lewis structure for the carbon dioxide (co2) molecule. assign a formal charge to each atom in the student's lewis structure atom formal charge x g 1eft o c right o instant answer. Click here 👆 to get an answer to your question ️ a student proposes the following lewis structure for the isocyanate (nco^ ) ion. [c=0=n ·s end(bmatrix)^. Your solution’s ready to go! our expert help has broken down your problem into an easy to learn solution you can count on. question: a student proposes the following lewis structure for the (nh4 )ion. assign a formal charge to each atom in the student's lewis structure. there are 2 steps to solve this one. Study with quizlet and memorize flashcards containing terms like a student proposes the following lewis structure for the nitronium no 2 ion. n o o assign a formal charge to each atom in the student's lewis structure., a student proposes the following lewis structure for the nitrate no−3 ion. n o o o assign a formal charge to each atom in the student's lewis structure., a student proposes.

Comments are closed.