Right Handed Alpha Helix
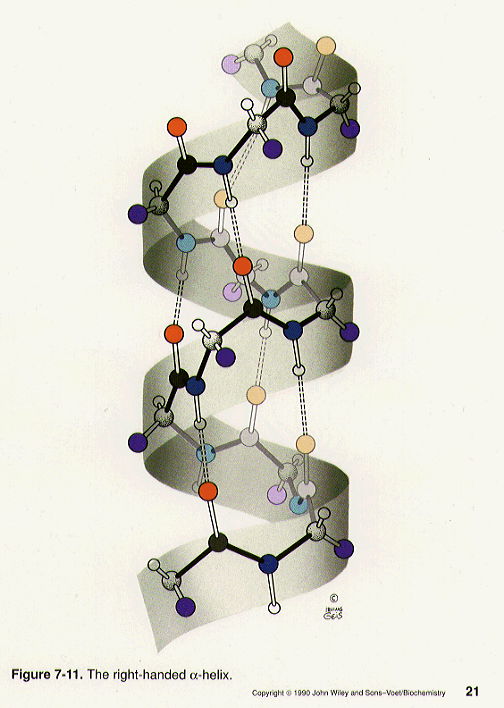
Right Handed Alpha Helix The alpha helix is the most common structural arrangement in the secondary structure of proteins. it is also the most extreme type of local structure, and it is the local structure that is most easily predicted from a sequence of amino acids. the alpha helix has a right handed helix conformation in which every backbone n−h group hydrogen. An α helix is a right handed coil of amino acid residues on a polypeptide chain, typically ranging between 4 and 40 residues. this coil is held together by hydrogen bonds between the oxygen of c=o on top coil and the hydrogen of n h on the bottom coil. such a hydrogen bond is formed exactly every 4 amino acid residues, and every complete turn.

Alpha Helix Right Handed Alpha helices are nearly all right handed. to see that this one is righthanded, hold your right hand with the thumb pointing up and the fingers loosely curled; trying to match the spiral of the helix, move slowly along the direction your thumb points and curl along the line of your fingers, as though tightening a screw. Why is that often when alpha helices are discussed, it is also mentioned their direction right handed (clockwise) or left handed (anti clockwise)? i have heard that left handed alpha helices are. The “screw sense” of an alpha helix can be right handed (clockwise) or left handed (counter clockwise). despite the fact that, based on the ramachandran plot, both right handed and left handed alpha helices are among the permitted conformations, the right handed alpha helix is energetically more favorable because of fewer steric clashes. In proteins, the alpha helix is chiral. it almost always adopts a right handed curl. if you start at one end of the helix, with your right thumb pointing along the chain from the end, the fingers of your right hand will follow the direction of the chain as it curls along the helix. in a left handed helix, the same thing is true with your left hand.

Ppt Chapter 4 The Three Dimensional Structure Of Proteins Powerpoint The “screw sense” of an alpha helix can be right handed (clockwise) or left handed (counter clockwise). despite the fact that, based on the ramachandran plot, both right handed and left handed alpha helices are among the permitted conformations, the right handed alpha helix is energetically more favorable because of fewer steric clashes. In proteins, the alpha helix is chiral. it almost always adopts a right handed curl. if you start at one end of the helix, with your right thumb pointing along the chain from the end, the fingers of your right hand will follow the direction of the chain as it curls along the helix. in a left handed helix, the same thing is true with your left hand. Proteins typically consist of right handed alpha helices, whereas left handed alpha helices are rare in nature. peptides of 20 amino acids or less corresponding to protein helices do not form thermodynamically stable alpha helices in water away from protein environments. the smallest known water stable right (αr) and left (αl) handed alpha helices are reported, each stabilized in cyclic. The α−helix is basically that of circular staircase with the polypeptide backbone winding in a right handed, stair step fashion from one amino acid aa residue to the next along its corresponding aa sequence, i.e., the primary structure. the symmetrical stair step motif of the α−helix classifies it as a secondary structure, i.e., a.
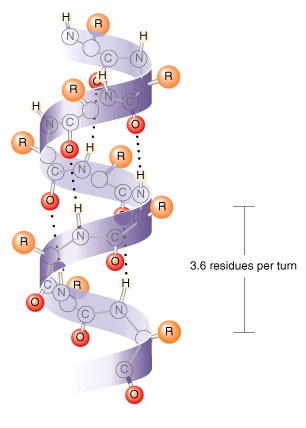
Gr09 05 Proteins typically consist of right handed alpha helices, whereas left handed alpha helices are rare in nature. peptides of 20 amino acids or less corresponding to protein helices do not form thermodynamically stable alpha helices in water away from protein environments. the smallest known water stable right (αr) and left (αl) handed alpha helices are reported, each stabilized in cyclic. The α−helix is basically that of circular staircase with the polypeptide backbone winding in a right handed, stair step fashion from one amino acid aa residue to the next along its corresponding aa sequence, i.e., the primary structure. the symmetrical stair step motif of the α−helix classifies it as a secondary structure, i.e., a.

Comments are closed.