Proteomics Chaperones In Protein Folding

Chaperone Aided Protein Folding Physical Lens On The Cell The chaperones that participate broadly in de novo protein folding and refolding, such as the hsp70s, hsp90s and the chaperonins (hsp60s), are multicomponent molecular machines that promote. To avoid these dangers, cells invest in a complex network of molecular chaperones, which use ingenious mechanisms to prevent aggregation and promote efficient folding. because protein molecules are highly dynamic, constant chaperone surveillance is required to ensure protein homeostasis (proteostasis).

Proteomics Chaperones In Protein Folding Youtube The sensitivity of the protein folding environment to chaperone disruption can be highly tissue specific. the mass spectrometry proteomics data were deposited in proteomexchange consortium via. Protein folding in vivo begins during synthesis on the ribosome and is modulated by molecular chaperones that engage the nascent polypeptide. we use proteomics, biophysical measurements and. Protein folding is a spontaneous process. however, under physiological conditions, proteins are inherently unstable, and the protein concentrations in living cells favor unspecific interactions between partially folded proteins. thus, the cellular proteome requires the assistance of helper factors, the molecular chaperones, for quality control and the maintenance of protein homeostasis. this. Abstract. the biological functions of proteins are governed by their three dimensional fold. protein folding, maintenance of proteome integrity, and protein homeostasis (proteostasis) critically depend on a complex network of molecular chaperones. disruption of proteostasis is implicated in aging and the pathogenesis of numerous degenerative.
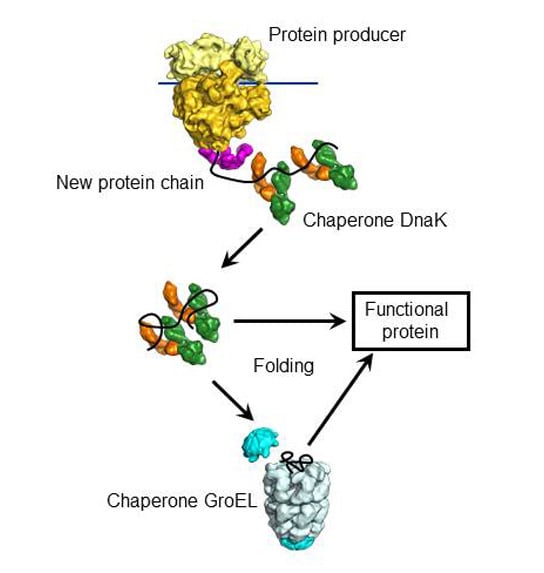
Dnak Identified As Key Player Of Protein Folding Protein folding is a spontaneous process. however, under physiological conditions, proteins are inherently unstable, and the protein concentrations in living cells favor unspecific interactions between partially folded proteins. thus, the cellular proteome requires the assistance of helper factors, the molecular chaperones, for quality control and the maintenance of protein homeostasis. this. Abstract. the biological functions of proteins are governed by their three dimensional fold. protein folding, maintenance of proteome integrity, and protein homeostasis (proteostasis) critically depend on a complex network of molecular chaperones. disruption of proteostasis is implicated in aging and the pathogenesis of numerous degenerative. 1. introduction. the unique tertiary and quaternary structure of a protein is critical for its function. under the right conditions of ph, temperature, solute concentration, intracellular ions and solvent, many proteins are capable of spontaneously folding due to the intramolecular forces of the amino acids of the linear protein chain [1,2,3,4]. In bacteria, the major chaperones involved in co translational folding are trigger factor (tf), dnaj, and dnak (hsp40 and hsp70 in humans, respectively). most protein folding studies to date have focused on the folding of single domain proteins in the absence of folding factors. while these studies have revealed important insights into folding.

Comments are closed.