Protein Structure

Food Science And Technology Structure Of Protein Learn about the four levels of protein structure (primary, secondary, tertiary, and quaternary) and how they are determined by amino acid sequence, hydrogen bonds, and non covalent interactions. explore the concepts of domains, motifs, and folds in protein structure and the techniques of structural biology. Amino acid structure. amino acids are the monomers that make up proteins. each amino acid has the same core structure, which consists of a central carbon atom, also known as the alpha (α) carbon, bonded to an amino group (nh2), a carboxyl group (cooh), and a hydrogen atom. every amino acid also has another atom or group of atoms bonded to the.

Lecture Notes In Medical Technology Lecture 10 Proteins There are two general classes of protein molecules: globular proteins and fibrous proteins. globular proteins are generally compact, soluble, and spherical in shape. fibrous proteins are typically elongated and insoluble. globular and fibrous proteins may exhibit one or more of four types of protein structure. bailey, regina. (2024, july 26). Learn about the four levels of protein structure (primary, secondary, tertiary, and quaternary) and how they determine the function of proteins. also, find out the difference between globular and fibrous proteins and what is protein denaturation. Learn how proteins are made of amino acids and how they fold into different shapes and conformations. explore the methods and examples of protein structure analysis and the role of chaperone proteins in folding. The detailed structure of any protein is complicated; for simplicity a protein’s structure can be depicted in several different ways, each emphasizing different features of the protein. panel 3 2 (pp. 138–139) presents four different depictions of a protein domain called sh2, which has important functions in eucaryotic cells.
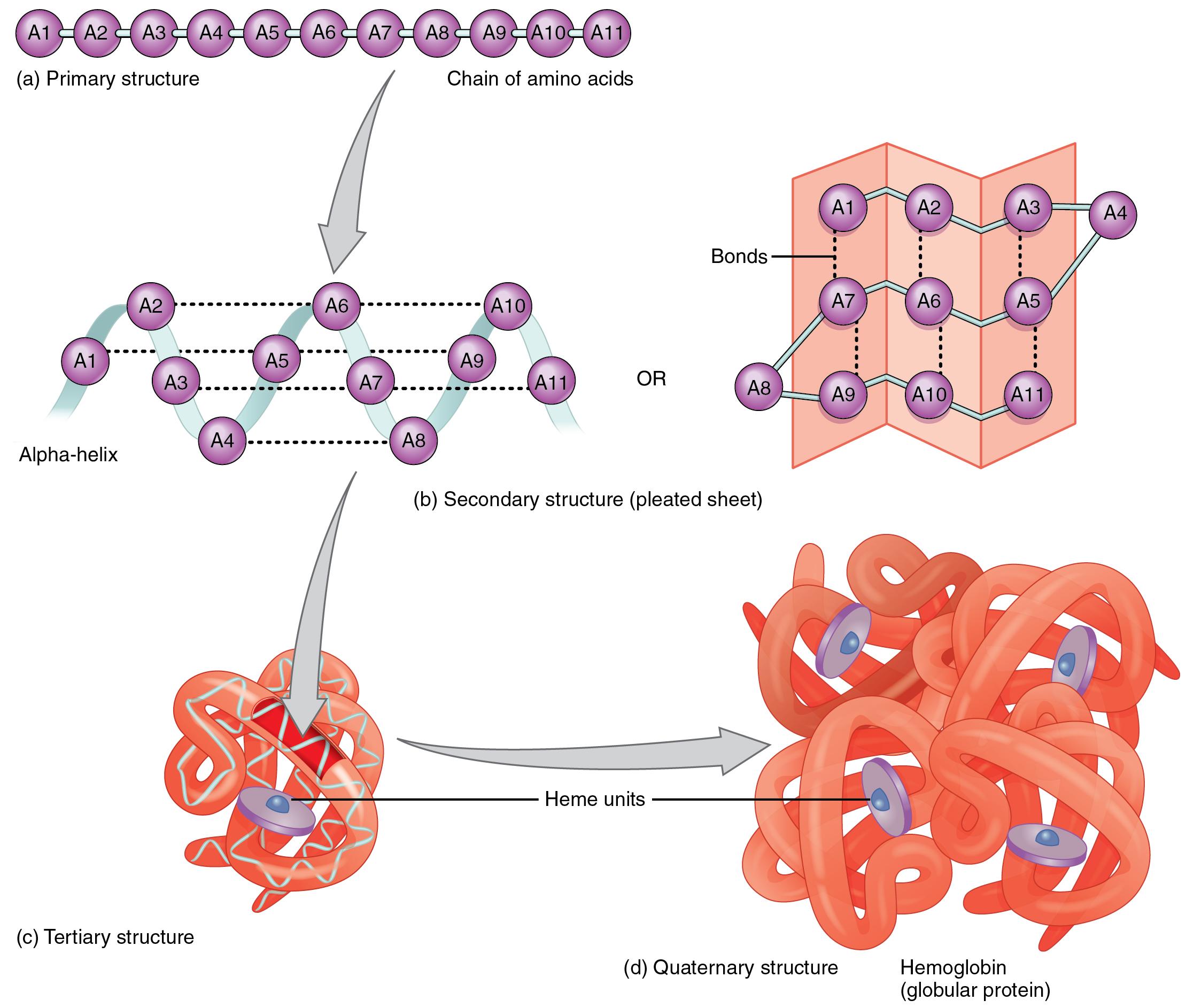
2 23 Protein Structure Nutrition Flexbook Learn how proteins are made of amino acids and how they fold into different shapes and conformations. explore the methods and examples of protein structure analysis and the role of chaperone proteins in folding. The detailed structure of any protein is complicated; for simplicity a protein’s structure can be depicted in several different ways, each emphasizing different features of the protein. panel 3 2 (pp. 138–139) presents four different depictions of a protein domain called sh2, which has important functions in eucaryotic cells. The complete structure of a protein can be described at four different levels of complexity: primary, secondary, tertiary, and quaternary structure. as a multitude of protein structures are rapidly being determined by x‐ray crystallography and by nuclear magnetic resonance (nmr), it is becoming clear that the number of unique folds is far less than the total number of protein structures. Orders of protein structure: primary, secondary, tertiary, and quaternary. alpha helix and beta pleated sheet.

Comments are closed.