Protein Folding Gamma
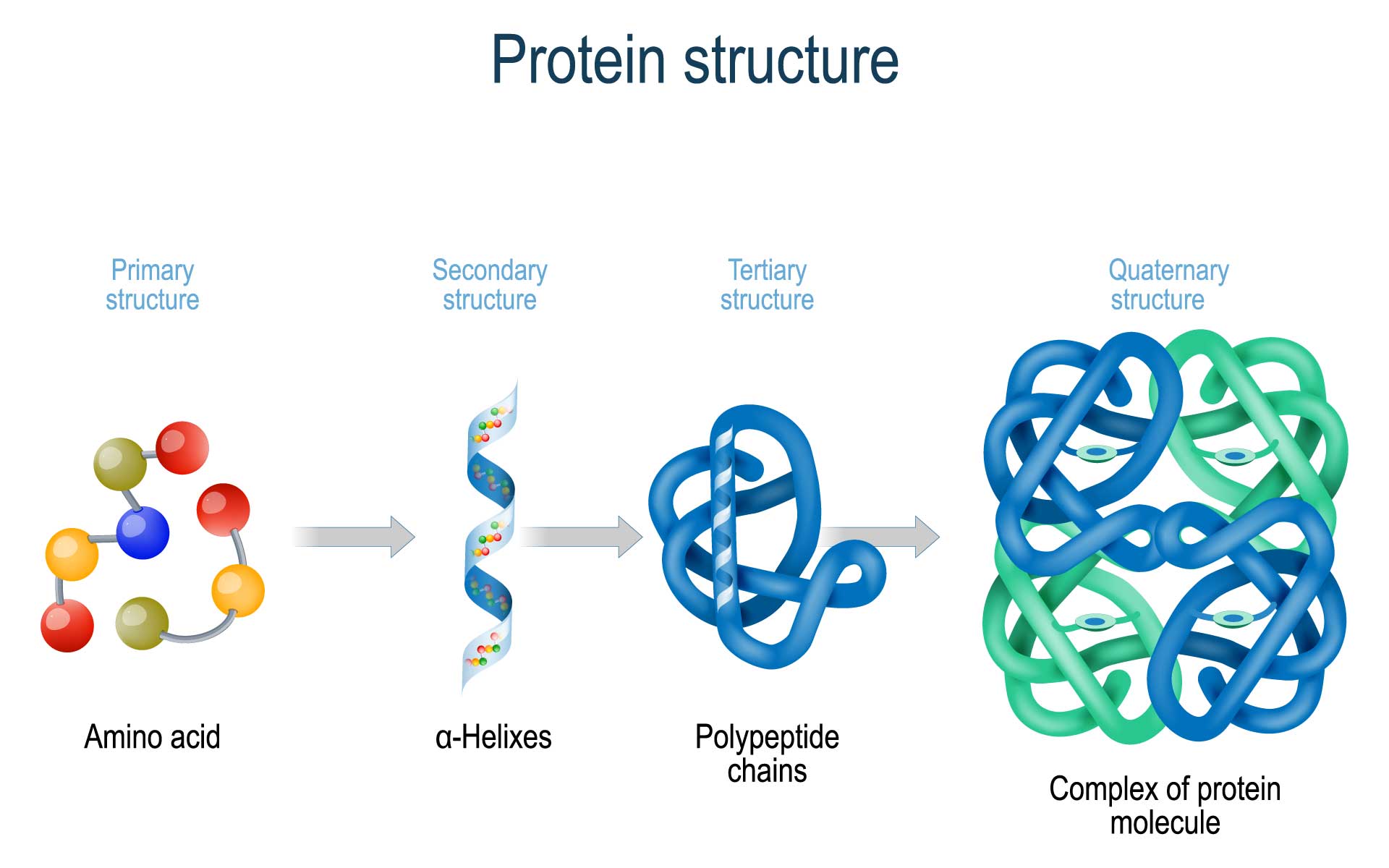
Protein Folding Gamma The mechanism of folding for this family of proteins depends on the formation of the β hairpin with the lower free energy at the transition state, making it possible to inter convert the folding pathway of proteins g and l by switching the intrinsic turn propensities of the two hairpins. 77 a redesigned protein g mutant was constructed by. Amino acid structure. amino acids are the monomers that make up proteins. each amino acid has the same core structure, which consists of a central carbon atom, also known as the alpha (α) carbon, bonded to an amino group (nh2), a carboxyl group (cooh), and a hydrogen atom. every amino acid also has another atom or group of atoms bonded to the.

Scheme 1 Schematic Protein Folding In The Presence Of Pmdp оі Fe 2 O 3 Our model successfully predicts protein folding processes consistent with experiments, without the limitations of protein size and shape. {\alpha,\beta,\gamma,\delta }\), should be multiplied. Protein folding. proteins are folded and held together by several forms of molecular interactions. the molecular interactions include the thermodynamic stability of the complex, the hydrophobic interactions and the disulfide bonds formed in the proteins. the figure below (figure 2 2) is an example of protein folding. Watch on. foldit is a revolutionary crowdsourcing computer game enabling you to contribute to scientific research. learn the science behind foldit and how your playing can help. about foldit start playing. Tertiary structure describes the folding of the polypeptide chain to assemble the different secondary structure elements in a particular arrangement. as helices and sheets are units of secondary structure, so the domain is the unit of tertiary structure. in multi domain proteins, tertiary structure includes the arrangement of domains relative.

The Protein Folding Problem 50 Years On Science Watch on. foldit is a revolutionary crowdsourcing computer game enabling you to contribute to scientific research. learn the science behind foldit and how your playing can help. about foldit start playing. Tertiary structure describes the folding of the polypeptide chain to assemble the different secondary structure elements in a particular arrangement. as helices and sheets are units of secondary structure, so the domain is the unit of tertiary structure. in multi domain proteins, tertiary structure includes the arrangement of domains relative. Folding rate as a function of the geometry and topology of the native state for a mixed (two state and multi state) set of proteins. figure 4 shows the logarithm of the experimental folding rate. The correct folding is a key process for a protein to acquire its functional structure and conformation. prefoldin is a well known chaperone protein that regulates the correct folding of proteins. prefoldin plays a crucial role in the pathogenesis of common neurodegenerative diseases (alzheimer’s disease, parkinson’s disease, and huntington’s disease). the important role of prefoldin in.

Comments are closed.