Ppt Theories Of Chemical Bonding Powerpoint Presentation Free
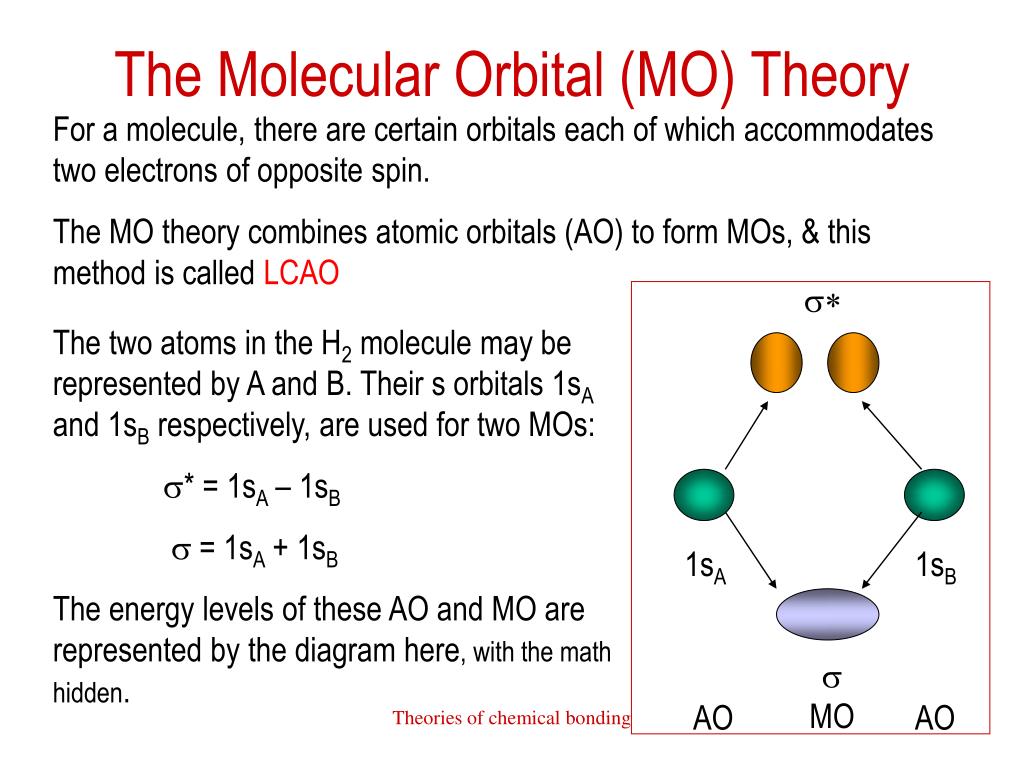
Ppt Theories Of Chemical Bonding Powerpoint Presentation Free Ionic bonding: summary. an ionic bond is the electrostatic attraction formed between ( ) cations and ( ) anions. the cations and anions are formed when a non metal (high en) steals an electron from a metal (low ie). this transfer of electrons gives each atom a filled valence shell (stable octet) of electrons. Theories of chemical bonding. bonding of h2c=ch2 molecules utilizing the sp2 hybrid orbitals, each c atom form two h–c s bonds for a total of 4 s h–c bonds. the c–c s bond is common to both c atoms. a c–c p bond is formed due to overlap of p orbitals from each of the c atoms.

Ppt Theories Of Chemical Bonding Powerpoint Presentation Free About this presentation. title: theories of chemical bonding. description: theories of chemical bonding theories of bonding: explanations for chemical bond, lewis dot structures and the following. valance bond (vb) theory – powerpoint ppt presentation. number of views: 60. avg rating:3.0 5.0. slides: 37. Help. view only. 1 chemical bonding chapter 7 2 the octet rule atoms tend to gain, lose, or share electrons in order to get a full set of valence electrons. “octet” – most atoms need 8 valence electrons for a full set gaining or losing → ions = ionic bonding sharing = covalent bonding “dogs teaching chemistry”. Follow. 1) the presentation summarizes atomic structure and chemical bonding models including fundamental particles, rutherford and bohr atomic models, quantum numbers, and types of chemical bonds. 2) it discusses limitations of earlier atomic models and introduces concepts like electron energy levels, photon energy, and spectral lines. Packing of the ion only. charge and size of the ion. element x is strongly electropositive and y is strongly electronegative. both are univalent. then the formula of the compound formed would be: x y . x y. x y . x y.

Ppt Theories Of Chemical Bonding Powerpoint Presentation Free Follow. 1) the presentation summarizes atomic structure and chemical bonding models including fundamental particles, rutherford and bohr atomic models, quantum numbers, and types of chemical bonds. 2) it discusses limitations of earlier atomic models and introduces concepts like electron energy levels, photon energy, and spectral lines. Packing of the ion only. charge and size of the ion. element x is strongly electropositive and y is strongly electronegative. both are univalent. then the formula of the compound formed would be: x y . x y. x y . x y. 670 likes | 1k views. theories of chemical bonding. exam #4 (chapter 8) on 7 december one 3” x 5” notecard chapter 8 owl deadline tuesday night chapter 9 owl deadline @ final exam f inal exam (review session weekend before) monday, 12 december @ 8am in irc 3 (here) ~50 points just on chapter 9. download presentation. energy level diagrams. Alkenes. 2. methene does not exist because alkenes have a double bond between two carbon atoms – methane would have only one carbon atom. 3. when one carbon atom is added to the chain, the number of hydrogen atoms increases by two. the number of hydrogen atoms is double the number of carbon atoms. 4.

Comments are closed.