Pps 97 Alpha Helix Geometry Part 1
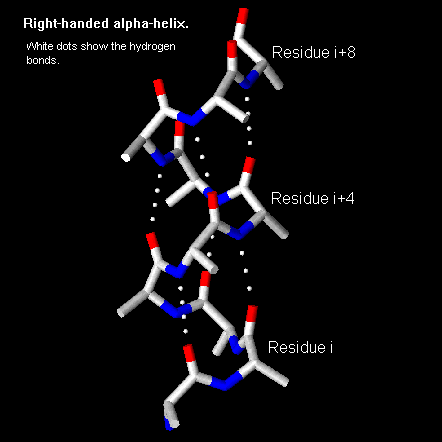
Pps 97 Alpha Helix Geometry Part 1 Development of a model for alpha helix structure. pauling and corey twisted models of polypeptides around to find ways of getting the backbone into regular conformations which would agree with alpha keratin fibre diffraction data. the most simple and elegant arrangement is a right handed spiral conformation known as the 'alpha helix'. Nature (1983) 306, 281 283. `helix geometry in proteins', d.barlow and j.thornton. j.molec.biol. (1988) 201, 601 619. `conformation of beta hairpins in protein structures. a systematic classification with applications to modelling by homology, electron density fitting and protein engineering', b.l.sibanda and j.m.thornton.
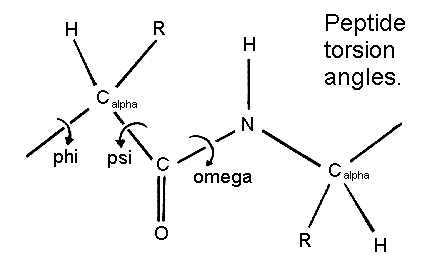
Alpha Helix Geometry Part 1 The structure repeats itself every 5.4 angstroms along the helix axis, ie we say that the alpha helix has a pitch of 5.4 angstroms. alpha helices have 3.6 amino acid residues per turn, ie a helix 36 amino acids long would form 10 turns. the separation of residues along the helix axis is 5.4 3.6 or 1.5 angstroms, ie the alpha helix has a rise. The resulting functional relations also show unexpected behaviour. for a typically observed alpha helix (omega = 99.1 degrees, delta = 1.45 a), the three optimal packing angles are omega a,b,c = 37.1 degrees, 97.4 degrees and 22.0 degrees with a periodicity of 180 degrees and respective helix radii ra,b,c = 3.0 a, 3.5 a and 4.3 a. α helix and coiled coil geometry. a, the α helix has a rise per residue (r) of 1.5 Å, 3.6 residues per helical turn, a backbone radius of 2.3 Å, and is stabilized by i co to i 4 nh hydrogen bonds. b, in crick’s helical nets, the positions of the cα atoms of an α helix, are projected as points onto a 2d plot (red). J. = 1. i = j. i . where n is the number of residues in a protein chain, i residue index, and πt a threshold for helix score π and ai a threshold for helix axis angle used only in π helix assignment. next the algorithm assigns 3 10 helices for the remaining residues of the same protein chain. while i < n and b.
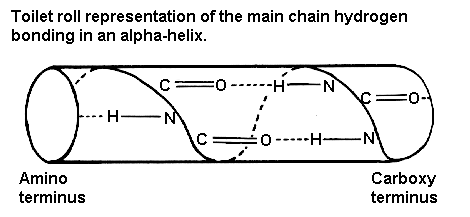
Pps 97 Alpha Helix Geometry Part 2 α helix and coiled coil geometry. a, the α helix has a rise per residue (r) of 1.5 Å, 3.6 residues per helical turn, a backbone radius of 2.3 Å, and is stabilized by i co to i 4 nh hydrogen bonds. b, in crick’s helical nets, the positions of the cα atoms of an α helix, are projected as points onto a 2d plot (red). J. = 1. i = j. i . where n is the number of residues in a protein chain, i residue index, and πt a threshold for helix score π and ai a threshold for helix axis angle used only in π helix assignment. next the algorithm assigns 3 10 helices for the remaining residues of the same protein chain. while i < n and b. The geometry of an alpha helix is characterized by computing local helix axes and local helix origins for four contiguous c alpha atoms, using the procedure of sugeta and miyazawa ([sm67]) and sliding this window over the length of the helix in steps of one c alpha atom. helanal computes a number of properties. The pdb format supports 10 different types of helix, but only three of these (alpha, pi, and 3 10) are common in naturally occurring proteins and have easily deciphered hydrogen bonding rules. user specified helices default to alpha form (hydrogen bonds between residue n and residue n 4). sheets may be either parallel or antiparallel.
Pps 97 Alpha Helix Geometry Part 1 The geometry of an alpha helix is characterized by computing local helix axes and local helix origins for four contiguous c alpha atoms, using the procedure of sugeta and miyazawa ([sm67]) and sliding this window over the length of the helix in steps of one c alpha atom. helanal computes a number of properties. The pdb format supports 10 different types of helix, but only three of these (alpha, pi, and 3 10) are common in naturally occurring proteins and have easily deciphered hydrogen bonding rules. user specified helices default to alpha form (hydrogen bonds between residue n and residue n 4). sheets may be either parallel or antiparallel.

Alpha Helix Geometry Part 1

Comments are closed.