Physiology Saq19 Haemoglobin

Physiology Saq19 Haemoglobin Youtube A) describe the structure of adult (hba) and foetal haemoglobin [3marks]b) describe the molecular mechanism of oxygen binding by haemoglobin. [3 marks].c) wh. Structure and function of hemoglobin. the primary function of hb is to transport oxygen (o 2) from the lung to tissues, binding and releasing o 2 in a cooperative manner, as demonstrated by the oxygen equilibrium curve (oec), which represents o 2 saturation of hb (so 2) at varying partial pressures of o 2 (po 2) (fig. 14.1).
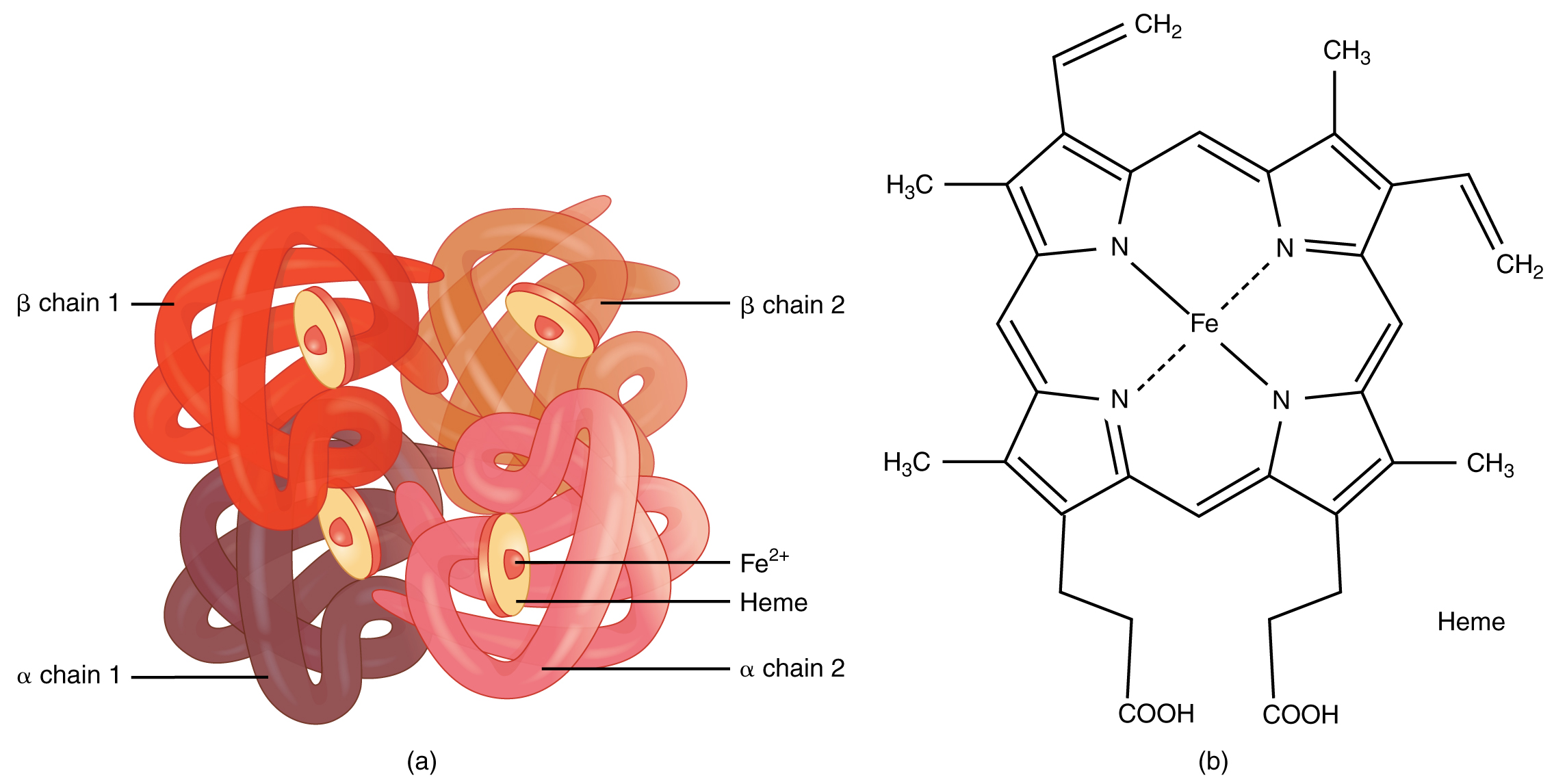
Erythrocytes в Anatomy And Physiology Haemoglobin is a heterotetramer protein composed of four subunits, two α and two β. its quaternary structure changes with oxygen binding to increase its affinity for oxygen. at the core is a haem molecule, which contains iron and which performs essential gas transport and redox functions. additionally, haemoglobin functions as a carrier for co2 and a buffer for the extracellular fluid. Of an adult's haemoglobin, 2.2–3.5% is hba 2, composed of two α and two δ chains. this form of haemoglobin is poor at oxygen carriage. fetal haemoglobin (hbf) comprises two α chains and two γ chains. at birth, 50–95% of a baby's haemoglobin is hbf, but these levels decline after 6 months as more hba is produced. Oxygen is primarily transported throughout the body in red blood cells, attached to hemoglobin molecules. oxygen is also dissolved directly in the bloodstream, but this dissolved fraction contributes little to the total amount of oxygen carried in the bloodstream. henry’s law states that the dissolved fraction is proportional to the atmospheric po2, but the solubility of oxygen is so low. A shift to the left indicates increased hemoglobin affinity for oxygen and an increased reluctance to release oxygen. several physiologic factors are responsible for shifting the curve left or right, such as ph, carbon dioxide (co2), temperature, and 2,3 disphosphoglycerate. ph. a decrease in ph (acidity) shifts the dissociation curve to the.

Hemoglobin Anatomy Oxygen is primarily transported throughout the body in red blood cells, attached to hemoglobin molecules. oxygen is also dissolved directly in the bloodstream, but this dissolved fraction contributes little to the total amount of oxygen carried in the bloodstream. henry’s law states that the dissolved fraction is proportional to the atmospheric po2, but the solubility of oxygen is so low. A shift to the left indicates increased hemoglobin affinity for oxygen and an increased reluctance to release oxygen. several physiologic factors are responsible for shifting the curve left or right, such as ph, carbon dioxide (co2), temperature, and 2,3 disphosphoglycerate. ph. a decrease in ph (acidity) shifts the dissociation curve to the. Once oxygen has entered the blood from the lungs, it can be bound by haemoglobin (hb) in the red blood cells. haemoglobin is a protein comprised of four subunits: two alpha subunits and two beta subunits. each subunit has a haem group in the centre that contains iron and binds one oxygen molecule. this means each haemoglobin molecule can bind. Oxygen (o 2) is an essential molecule in the human body. it is the final electron acceptor in the electron transport chain, located in the mitochondria, and so has a key role in the production of aerobic energy – i.e. adenosine triphosphate (atp). a constant supply is therefore required to tissues around the body, and this is achieved by the.

Comments are closed.