How To Calculate Formal Charge Lewis Structure

How To Calculate Formal Charge On A Lewis Structure The Tech Edvocate A step by step description on how to calculate formal charges. formal charges are important because they allow us to predict which lewis structure is the mo. To find formal charges in a lewis structure, for each atom, you should count how many electrons it "owns". count all of its lone pair electrons, and half of its bonding electrons. the difference between the atom's number of valence electrons and the number it owns is the formal charge. for example, in nh 3, n has 1 lone pair (2 electrons) and 3.

How To Calculate The Formal Charge Of An Atom Chemistry Youtube This chemistry video tutorial provides a basic introduction into how to calculate the formal charge of an atom or element in a lewis structure. the formal charge of an atom or element in a. Example \(\pageindex{1}\): calculating formal charge from lewis structures. assign formal charges to each atom in the interhalogen ion \(\ce{icl4 }\). s olution. we divide the bonding electron pairs equally for all \(\ce{i–cl}\) bonds: we assign lone pairs of electrons to their atoms. each cl atom now has seven electrons assigned to it, and. Formal charge. 10.7: formal charges is shared under a not declared license and was authored, remixed, and or curated by libretexts. in a lewis structure, formal charges can be assigned to each atom by treating each bond as if one half of the electrons are assigned to each atom. these hypothetical formal charges are a guide to …. 1. formal charge. formal charge is a book keeping formalism for assigning a charge to a specific atom to obtain the formal charge of an atom, we start by counting the number of valence electrons [note 1] for the neutral atom, and then subtract from it the number of electrons that it “owns” (i.e. electrons in lone pairs, or singly occupied orbitals) and half of the electrons that it.

Calculating Formal Charge Definition Formula Video Lesson Formal charge. 10.7: formal charges is shared under a not declared license and was authored, remixed, and or curated by libretexts. in a lewis structure, formal charges can be assigned to each atom by treating each bond as if one half of the electrons are assigned to each atom. these hypothetical formal charges are a guide to …. 1. formal charge. formal charge is a book keeping formalism for assigning a charge to a specific atom to obtain the formal charge of an atom, we start by counting the number of valence electrons [note 1] for the neutral atom, and then subtract from it the number of electrons that it “owns” (i.e. electrons in lone pairs, or singly occupied orbitals) and half of the electrons that it. Calculating formal charge from lewis structures assign formal charges to each atom in the interhalogen molecule brcl 3. solution. step 1. assign one of the electrons in each br–cl bond to the br atom and one to the cl atom in that bond: step 2. assign the lone pairs to their atom. now each cl atom has seven electrons and the br atom has seven. The formal charge of an atom in a molecule is the charge that would reside on the atom if all of the bonding electrons were shared equally. we can calculate an atom's formal charge using the equation fc = ve [lpe ½(be)], where ve = the number of valence electrons on the free atom, lpe = the number of lone pair electrons on the atom in the molecule, and be = the number of bonding (shared.
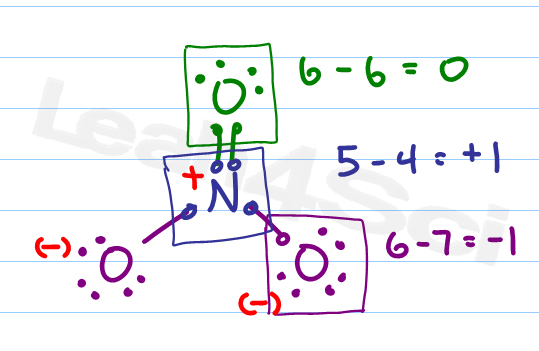
Formal Charge Formula Calculation And Shortcut For Organic Chemistry Calculating formal charge from lewis structures assign formal charges to each atom in the interhalogen molecule brcl 3. solution. step 1. assign one of the electrons in each br–cl bond to the br atom and one to the cl atom in that bond: step 2. assign the lone pairs to their atom. now each cl atom has seven electrons and the br atom has seven. The formal charge of an atom in a molecule is the charge that would reside on the atom if all of the bonding electrons were shared equally. we can calculate an atom's formal charge using the equation fc = ve [lpe ½(be)], where ve = the number of valence electrons on the free atom, lpe = the number of lone pair electrons on the atom in the molecule, and be = the number of bonding (shared.
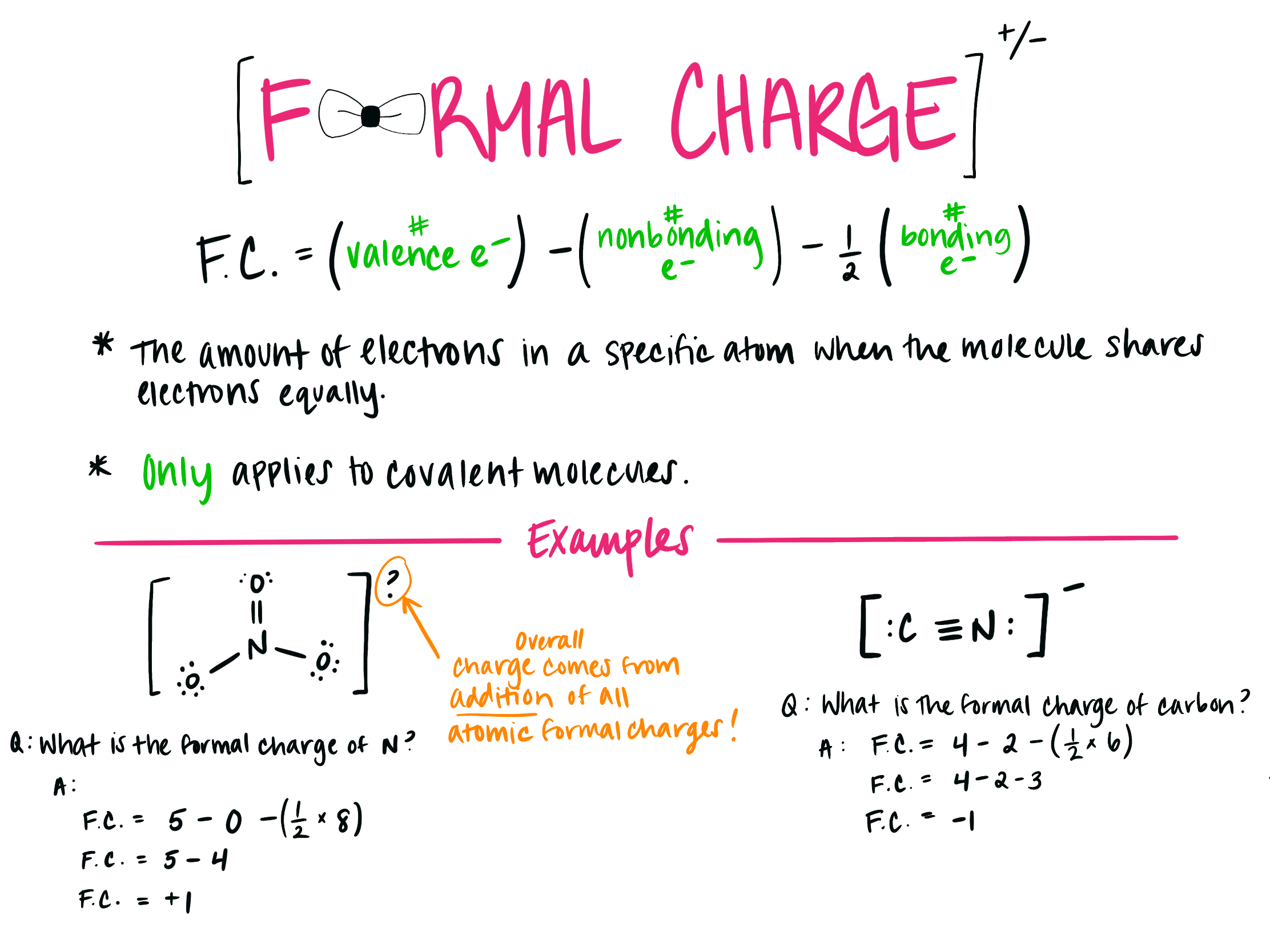
How To Calculate Formal Charge Lewis Structure

Comments are closed.