Hemoglobin Structure And Types
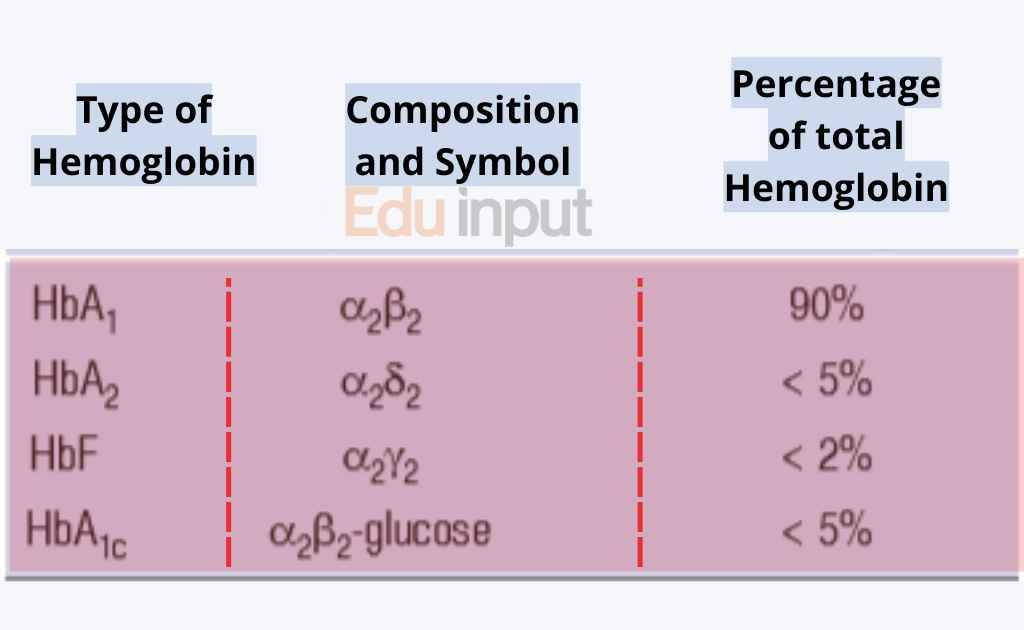
Types Of Hemoglobin With Structure And Functions Structure and function of hemoglobin. the primary function of hb is to transport oxygen (o 2) from the lung to tissues, binding and releasing o 2 in a cooperative manner, as demonstrated by the oxygen equilibrium curve (oec), which represents o 2 saturation of hb (so 2) at varying partial pressures of o 2 (po 2) (fig. 14.1). Hemoglobin: structure, types, functions, diseases. hemoglobin is a complex iron containing protein found in erythrocytes (red blood cells) of most vertebrates that plays a vital role in carrying oxygen and carbon dioxide within the blood. it is also the component that makes our blood red.
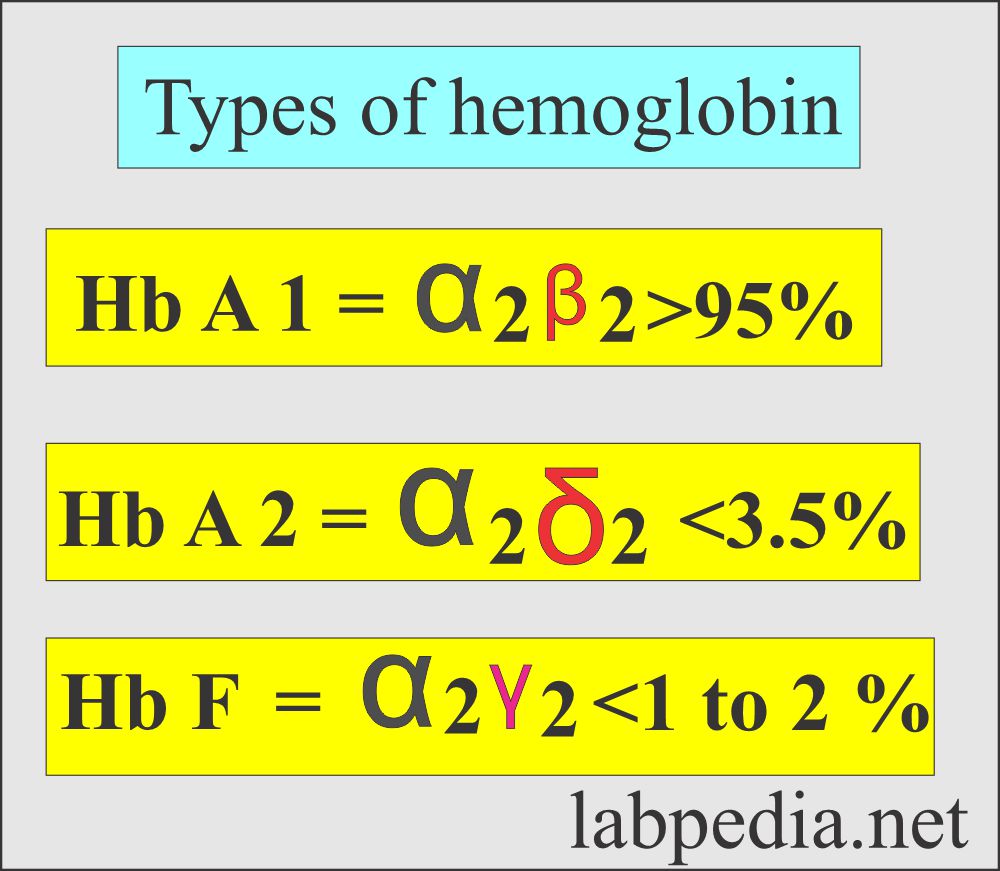
Hemoglobin Part 1 Hemoglobin Hb Structure And Functions Hemoglobin in the blood carries oxygen from the respiratory organs (lungs or gills) to the other tissues of the body, where it releases the oxygen to enable aerobic respiration which powers the animal's metabolism. a healthy human has 12 to 20 grams of hemoglobin in every 100 ml of blood. hemoglobin is a metalloprotein, a chromoprotein, and. Hemoglobin e. hemoglobin, iron containing protein in the blood of many animals—in the red blood cells (erythrocytes) of vertebrates —that transports oxygen to the tissues. hemoglobin forms an unstable reversible bond with oxygen. in the oxygenated state, it is called oxyhemoglobin and is bright red; in the reduced state, it is purplish blue. Hemoglobin is an iron containing protein in red blood cells (rbcs) that gives blood its red color. it has two primary functions: it transfers oxygen from your lungs to tissues throughout your body, and it carries carbon dioxide from cells back to the lungs so it can be expelled. when hemoglobin is too low, it can indicate certain types of. Hemoglobin is an oxygen binding protein found in erythrocytes that transports oxygen from the lungs to tissues. each hemoglobin molecule is a tetramer made of four polypeptide globin chains. each globin subunit contains a heme moiety formed of an organic protoporphyrin ring and a central iron ion in the ferrous state (fe2 ). the iron molecule in each heme moiety can bind and unbind oxygen.
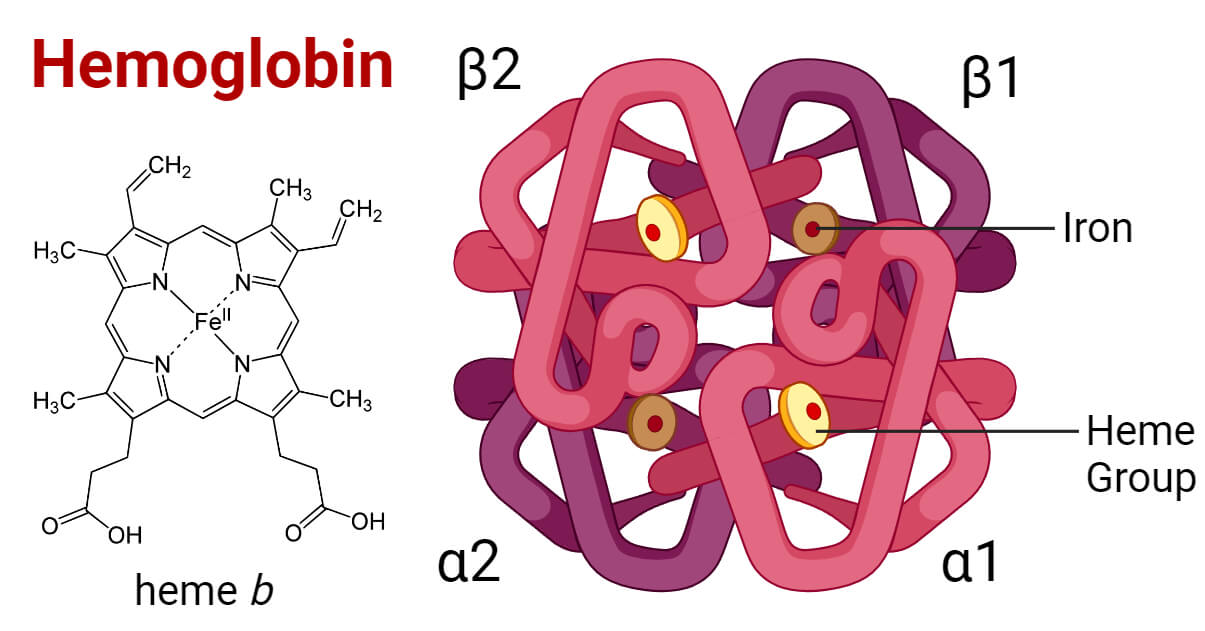
Hemoglobin Structure Types Functions Diseases Hemoglobin is an iron containing protein in red blood cells (rbcs) that gives blood its red color. it has two primary functions: it transfers oxygen from your lungs to tissues throughout your body, and it carries carbon dioxide from cells back to the lungs so it can be expelled. when hemoglobin is too low, it can indicate certain types of. Hemoglobin is an oxygen binding protein found in erythrocytes that transports oxygen from the lungs to tissues. each hemoglobin molecule is a tetramer made of four polypeptide globin chains. each globin subunit contains a heme moiety formed of an organic protoporphyrin ring and a central iron ion in the ferrous state (fe2 ). the iron molecule in each heme moiety can bind and unbind oxygen. This topic discusses the structure and function of the normal human hemoglobins, the main component of red blood cells, which are responsible for oxygen delivery. separate topic reviews discuss fetal hemoglobin (hb f) and abnormal hemoglobin variants associated with clinical disorders: hb f – (see "fetal hemoglobin (hb f) in health and disease".). Abstract. human hemoglobin (hb) is the erythrocyte hemeprotein resulting from the combination of one pair of α like (α or ζ) chains and another pair of β like (β, δ, γ or ε) chains. each of these chains is associated with a heme prosthetic group, a tetrapyrrole ring (protoporphyrin ix) containing a central ferrous atom (fe 2 ), which.
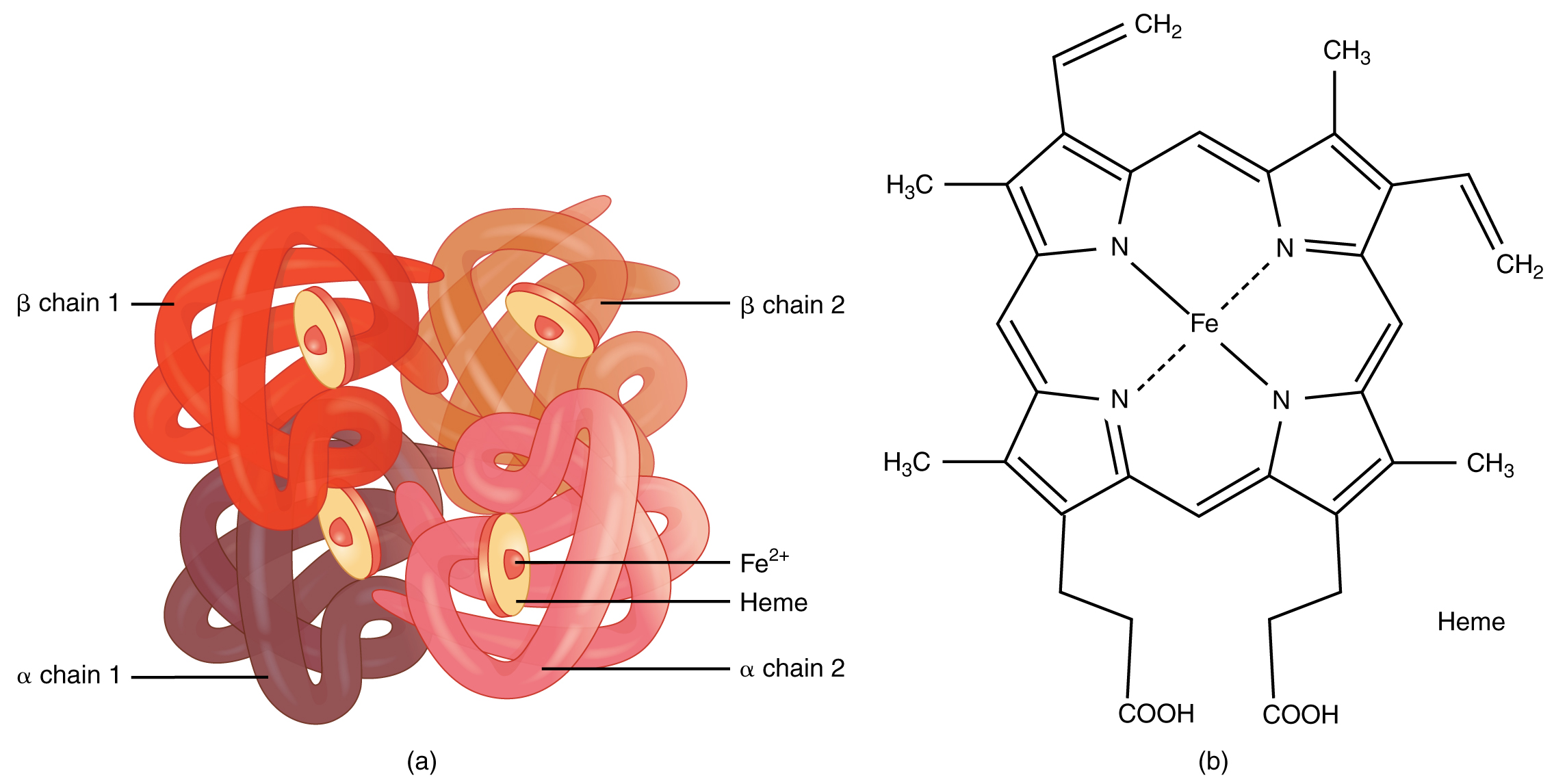
Erythrocytes Anatomy And Physiology This topic discusses the structure and function of the normal human hemoglobins, the main component of red blood cells, which are responsible for oxygen delivery. separate topic reviews discuss fetal hemoglobin (hb f) and abnormal hemoglobin variants associated with clinical disorders: hb f – (see "fetal hemoglobin (hb f) in health and disease".). Abstract. human hemoglobin (hb) is the erythrocyte hemeprotein resulting from the combination of one pair of α like (α or ζ) chains and another pair of β like (β, δ, γ or ε) chains. each of these chains is associated with a heme prosthetic group, a tetrapyrrole ring (protoporphyrin ix) containing a central ferrous atom (fe 2 ), which.

Comments are closed.