Covid 19 Risk Assessment Rev 2 Update

Covid 19 Risk Assessment Rev 2 Update Do not establish an mdi of covid 19 when a person alleges that they are medically at high risk for covid 19 or if the evidence indicates a false positive viral test. if a person has an mdi of covid 19, use the existing diagnosis code 1360 – other infectious and parasitic disorders. Establish a process to identify and manage individuals with suspected or confirmed sars cov 2 infection. ensure everyone is aware of recommended ipc practices in the facility. post visual alerts (e.g., signs, posters) at the entrance and in strategic places (e.g., waiting areas, elevators, cafeterias).

Covid 19 The University Of Texas System While body fluids other than respiratory secretions have not been clearly implicated in transmission of covid 19, unprotected contact with other body fluids, including blood, stool, vomit, and urine, might put hcp at risk of covid 19. table 1 describes possible scenarios that can be used to assist with risk assessment. Hcp with even mild symptoms of covid 19 should be prioritized for viral testing with nucleic acid or antigen detection assays. when testing a person with symptoms of covid 19, negative results from at least one viral test indicate that the person most likely does not have an active sars cov 2 infection at the time the sample was collected. 9 20 2023: clarification on ba.2.86 risk assessment posted on 8 23 2023. the first risk assessment cdc released on ba.2.86 included the following sentence: "ba.2.86 may be more capable of causing infection in people who have previously had covid 19 or who have received covid 19 vaccines." the intent of this sentence was to raise the possibility. The department began publishing fhp guidance and policy to address covid 19 in january 2020. in february 2021, the secretary of defense directed the review of all guidance and policy memoranda previously issued for covid 19.5 the review was completed in april 2021, and subsequent updates align dod covid 19 policy and guidance with current task.

Our Updated Covid 19 Risk Assessment Florence Melly Community Primary 9 20 2023: clarification on ba.2.86 risk assessment posted on 8 23 2023. the first risk assessment cdc released on ba.2.86 included the following sentence: "ba.2.86 may be more capable of causing infection in people who have previously had covid 19 or who have received covid 19 vaccines." the intent of this sentence was to raise the possibility. The department began publishing fhp guidance and policy to address covid 19 in january 2020. in february 2021, the secretary of defense directed the review of all guidance and policy memoranda previously issued for covid 19.5 the review was completed in april 2021, and subsequent updates align dod covid 19 policy and guidance with current task. Assessing risks of surgery due to current or recent sars cov 2 infection should include assessment of absolute risk, because any increase in relative risk impacts most those with the highest pre existing absolute risk . patients should also be informed that a positive pre operative sars cov 2 test may trigger a review of the risks of proceeding. We recommend individualised risk assessment for patients with elective surgery planned within 7 weeks of sars cov 2 infection. surgical patients should have received pre operative covid 19 vaccination, with three doses wherever possible, with the last dose at least 2 weeks before surgery.

Covid 19 Risk Assessment Bernard Matthews Foods Limited Assessing risks of surgery due to current or recent sars cov 2 infection should include assessment of absolute risk, because any increase in relative risk impacts most those with the highest pre existing absolute risk . patients should also be informed that a positive pre operative sars cov 2 test may trigger a review of the risks of proceeding. We recommend individualised risk assessment for patients with elective surgery planned within 7 weeks of sars cov 2 infection. surgical patients should have received pre operative covid 19 vaccination, with three doses wherever possible, with the last dose at least 2 weeks before surgery.
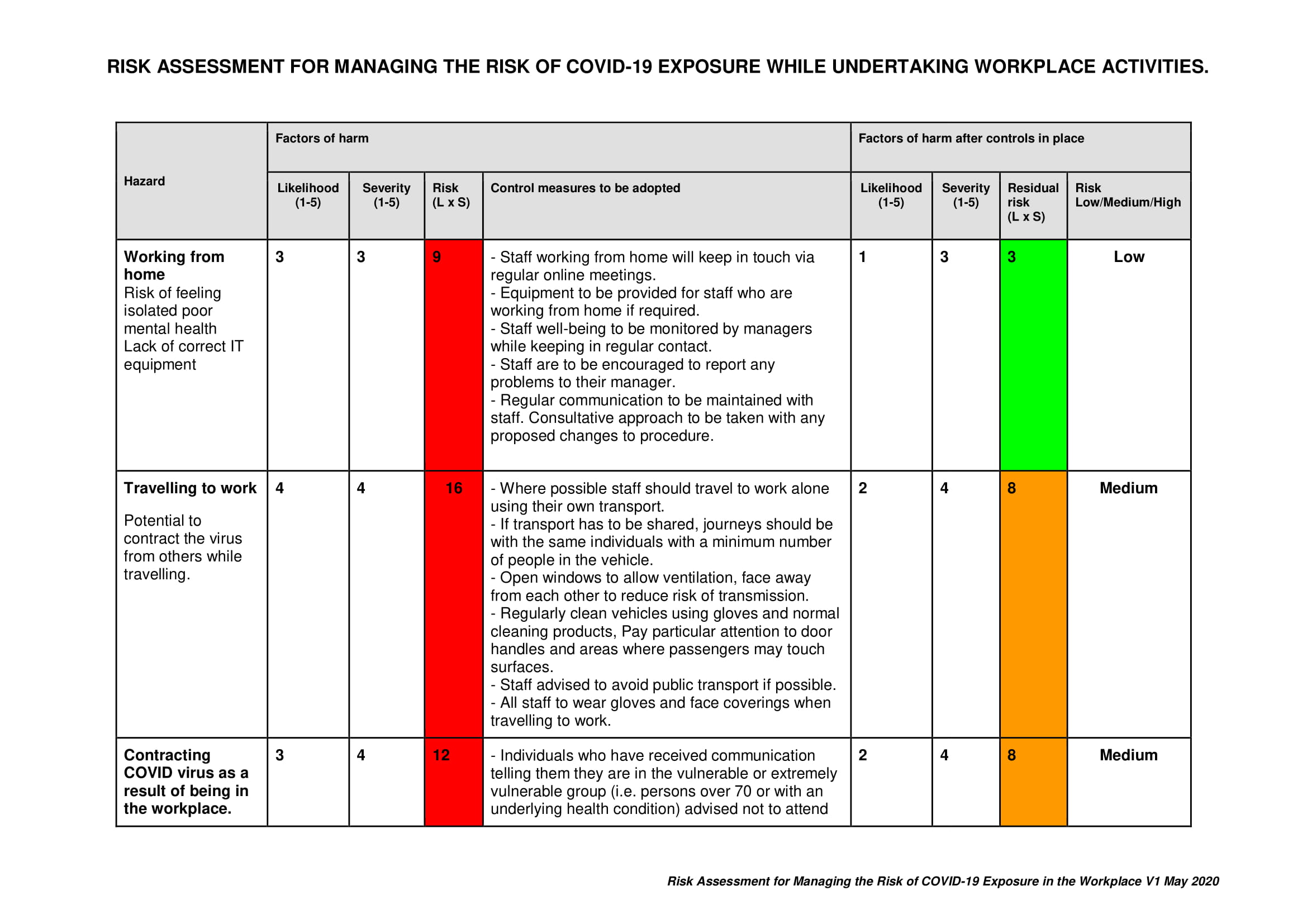
Pragnell Showroom Covid 19 Risk Assessment

Comments are closed.