Chaperones And Misfolded Proteins

Chaperone Mediated Degradation Of Misfolded Proteins Cma Is A Some soluble, misfolded proteins can bypass the refolding action of chaperones in vivo according to folding and functional assays 1,2,3.typically, in these assays the protein of interest is. Misfolded proteins can aggregate into larger structures, such as amyloid fibrils, which perpetuate the misfolding process, creating a self reinforcing cascade. notably, daxx, a polyd e protein.
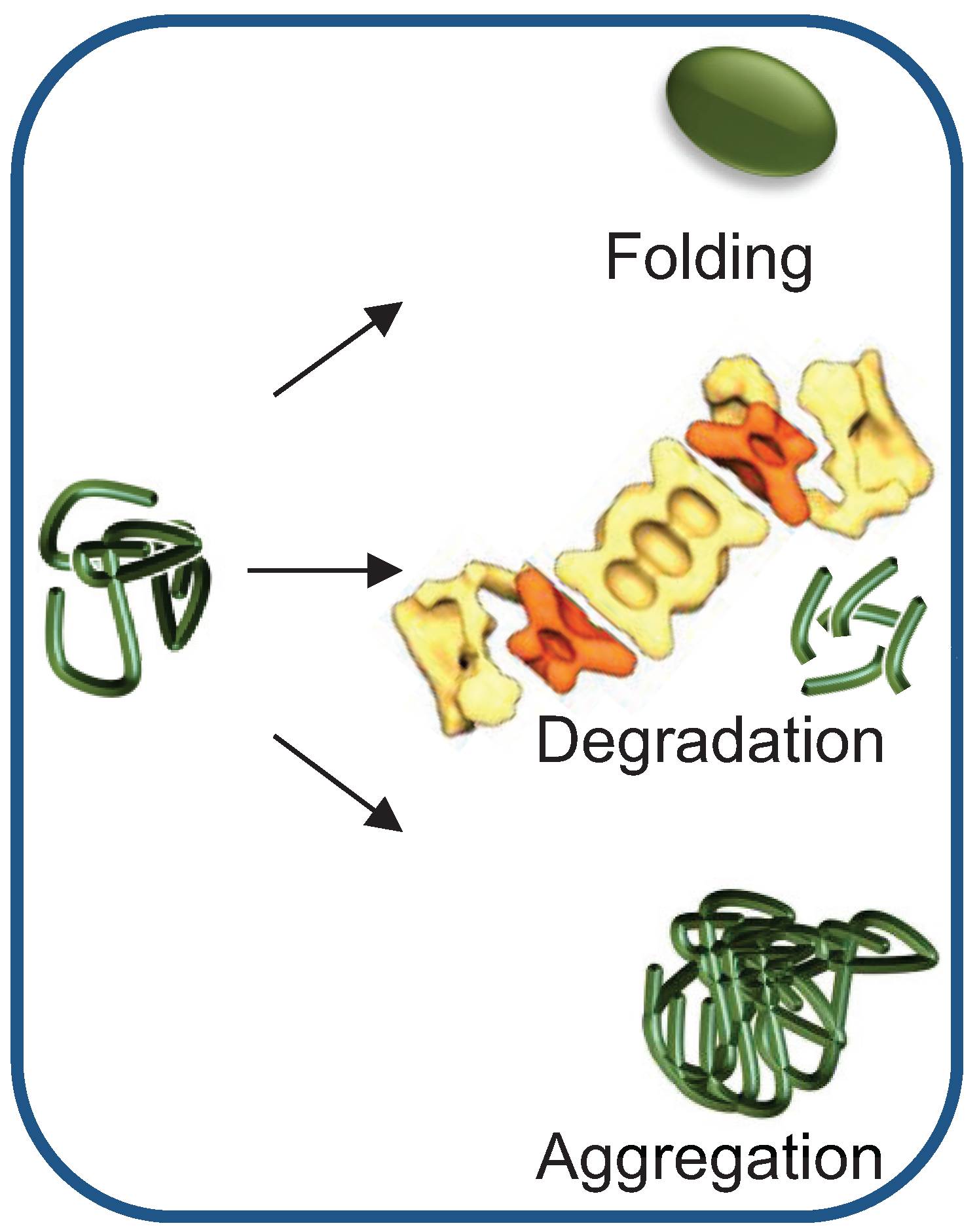
Schematic Representation Of The Fate Of Misfolded Proteins Within The In short, hsp70, hsp90, and their co chaperones are crucial members of the pn that are able to recognize misfolded proteins, aberrant condensates and protein aggregates, triaging proteins for refolding or degradation . this review will cover crucial aspects of the mechanisms for client recognition employed by hsp70 and hsp90 chaperones that. In general, cells rely on molecular chaperones to seize and refold misfolded proteins. if the native state is unattainable, misfolded proteins are targeted for degradation via the ubiquitin proteasome system. the specificity of this proteolysis is generally provided by e3 ubiquitin protein ligases, hundreds of which are encoded in the human genome. Interestingly, whereas the clearance of misfolded protein species by the ups requires that these molecules are maintained in a non aggregated state by chaperones, disposal by autophagy is thought. Molecular chaperones are central to protein homeostasis maintenance. cell chaperones not only guide newly synthesized polypeptides to their native structure, but they also help in the translocation of peptides and refolding of denatured intermediates. chaperones also target misfolded proteins towards proteasome machinery for degradation.

A Microscopic Image Of A Protein Aggregate With Molecular Chaperones Interestingly, whereas the clearance of misfolded protein species by the ups requires that these molecules are maintained in a non aggregated state by chaperones, disposal by autophagy is thought. Molecular chaperones are central to protein homeostasis maintenance. cell chaperones not only guide newly synthesized polypeptides to their native structure, but they also help in the translocation of peptides and refolding of denatured intermediates. chaperones also target misfolded proteins towards proteasome machinery for degradation. Misfolded proteins expose hydrophobic regions that promote formation of protein aggregates seen in many pathological states. to combat misfolding, cells arm themselves with chaperones to help fold proteins and maintain proteome solubility (balchin et al., 2016). with few exceptions, chaperoning was typically considered the domain of heat shock. Protein chaperones play a pivotal role in controlling protein quality and sustaining proteostasis through facilitating the accurate folding of nascent proteins and aiding in the refolding of misfolded proteins back to their native states. despite considerable advances in mapping out these protein networks since the discovery of chaperones, our molecular level understanding of how these.

Chaperones And Misfolded Proteins Youtube Misfolded proteins expose hydrophobic regions that promote formation of protein aggregates seen in many pathological states. to combat misfolding, cells arm themselves with chaperones to help fold proteins and maintain proteome solubility (balchin et al., 2016). with few exceptions, chaperoning was typically considered the domain of heat shock. Protein chaperones play a pivotal role in controlling protein quality and sustaining proteostasis through facilitating the accurate folding of nascent proteins and aiding in the refolding of misfolded proteins back to their native states. despite considerable advances in mapping out these protein networks since the discovery of chaperones, our molecular level understanding of how these.
Biomolecules Free Full Text Chaperoning Proteins For Destruction

Comments are closed.