Atomic Models Chemistry Ppt Download
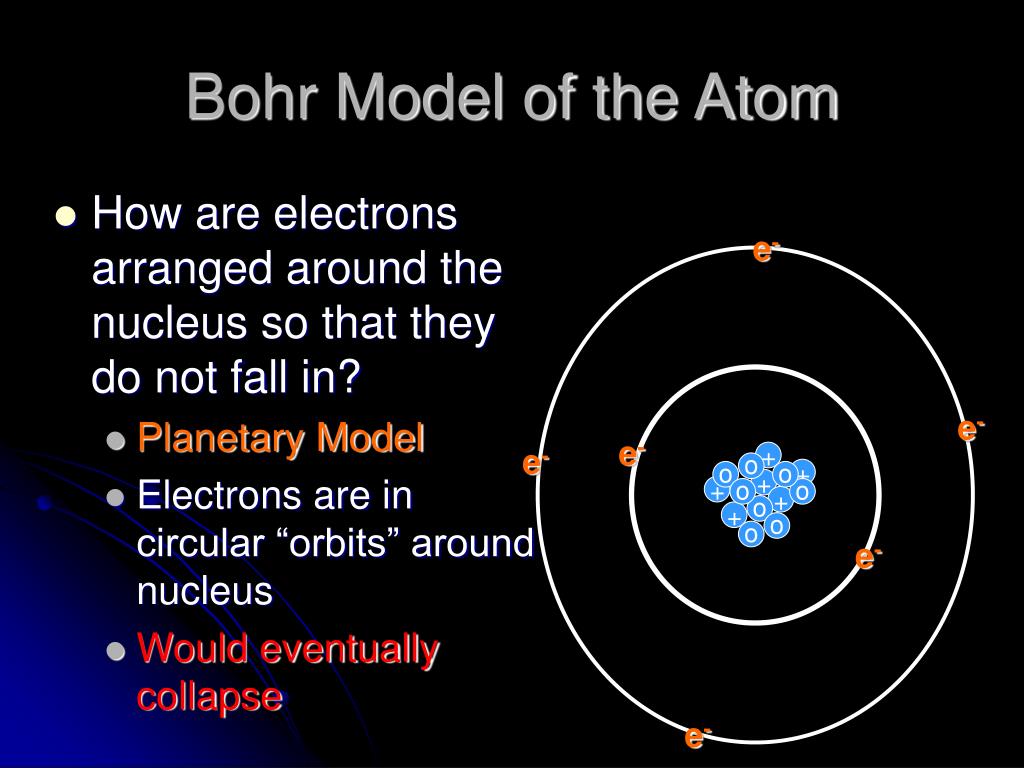
Ppt Bohr Model Of The Atom Powerpoint Presentation Free Download 1 of 12. download now. this document provides an outline for a presentation on the history and structure of atoms. it begins with the early ideas of democritus and thomson, then describes the atomic models of rutherford, bohr, and others. the structure of the atom is explained, including the nucleus, protons, neutrons, and electrons. Swbat determine the number of pen (protons, electrons and neutrons) in a neutral atom. 4 review what is an atom? • atom: the smallest unit of matter that retains the identity of the substance • first proposed by democratus 5 6 atomic structure • atoms are composed of 2 regions: 1.) nucleus the center of the atom that contains the mass.

What Are The Atomic Models Sir joseph john “j.j.”. thomson added the negatively charged electron in the model. 1856 1940 england. thomson. incorporated the idea of charges into an atomic model, now called the “raisin bun” model, shows negative charges. negative charges are randomly dispersed in positive surroundings. the charges could float around. Ohmiss. the document summarizes the development of atomic models over time from dalton's model to the current quantum mechanical model. dalton imagined atoms as spheres that differed in size and mass. thomson discovered the electron and proposed the "plum pudding" model. rutherford's gold foil experiment revealed the tiny, dense nucleus. Introduction to atomic theory ppt. dec 31, 2012 • download as pptx, pdf •. 17 likes • 24,109 views. ai enhanced description. mariana serrato. follow. the document summarizes the development of atomic theory over thousands of years through the proposals of different atomic models by scientists like democritus, dalton, thomson, rutherford. 210 likes | 361 views. atomic . models. around 400 bc a greek scientist . called democritus said that matter was . made up of small particles he named . 'atoma' (meaning indivisible). dalton 1803 round ball with a positive nucleus element made up of atoms that cannot be divided atoms of the same element are alike. download presentation.

Comments are closed.