Alpha Helix Geometry Part 1
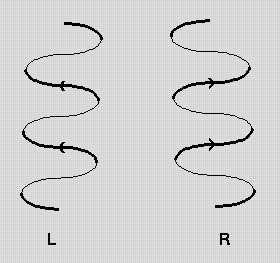
Pps 97 Alpha Helix Geometry Part 1 The figure below shows how a right handed helix differs from a left handed one. an easy way to remember this is to hold both your hands in front of you with your thumbs pointing up and your fingers curled towards you. for each hand the thumbs indicate the direction of translation and the fingers indicate the direction of rotation. j.cooper 2 1 95. Nature (1983) 306, 281 283. `helix geometry in proteins', d.barlow and j.thornton. j.molec.biol. (1988) 201, 601 619. `conformation of beta hairpins in protein structures. a systematic classification with applications to modelling by homology, electron density fitting and protein engineering', b.l.sibanda and j.m.thornton.

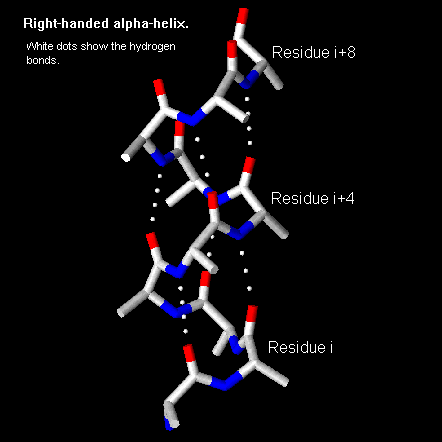
Alpha Helix Geometry Part 1 An α helix is a right handed coil of amino acid residues on a polypeptide chain, typically ranging between 4 and 40 residues. this coil is held together by hydrogen bonds between the oxygen of c=o on top coil and the hydrogen of n h on the bottom coil. such a hydrogen bond is formed exactly every 4 amino acid residues, and every complete turn. The pitch of the alpha helix (the vertical distance between consecutive turns of the helix) is 5.4 Å (0.54 nm), which is the product of 1.5 and 3.6. the most important thing is that the n h group of one amino acid forms a hydrogen bond with the c=o group of the amino acid four residues earlier; this repeated i 4 → i hydrogen bonding is the. Protein secondary structure is the local spatial conformation of the polypeptide backbone excluding the side chains. [ 1 ] the two most common secondary structural elements are alpha helices and beta sheets, though beta turns and omega loops occur as well. α helix and coiled coil geometry. a, the α helix has a rise per residue (r) of 1.5 Å, 3.6 residues per helical turn, a backbone radius of 2.3 Å, and is stabilized by i co to i 4 nh hydrogen bonds. b, in crick’s helical nets, the positions of the cα atoms of an α helix, are projected as points onto a 2d plot (red).
Pps 96 Alpha Helix Geometry Part 1 Protein secondary structure is the local spatial conformation of the polypeptide backbone excluding the side chains. [ 1 ] the two most common secondary structural elements are alpha helices and beta sheets, though beta turns and omega loops occur as well. α helix and coiled coil geometry. a, the α helix has a rise per residue (r) of 1.5 Å, 3.6 residues per helical turn, a backbone radius of 2.3 Å, and is stabilized by i co to i 4 nh hydrogen bonds. b, in crick’s helical nets, the positions of the cα atoms of an α helix, are projected as points onto a 2d plot (red). The values of the equilibrium states, x0 and x1, energy barriers, eb and er, and the transition state, xtr, are obtained from geometric analysis of the alpha helix geometry, as well as the full atomistic simulations. the transition state (local energy peak) corresponds to the breaking of hydrogen bonds between convolutions of the alpha helix. Two key developments in the modeling of the modern α helix were (1) the correct bond geometry, thanks to the crystal structure determinations of amino acids and peptides and pauling's prediction of planar peptide bonds; and (2) the relinquishing of the assumption of an integral number of residues per turn of the helix. the pivotal moment came.
Representative Geometry Of An Alpha Helix Structure A The Coiled The values of the equilibrium states, x0 and x1, energy barriers, eb and er, and the transition state, xtr, are obtained from geometric analysis of the alpha helix geometry, as well as the full atomistic simulations. the transition state (local energy peak) corresponds to the breaking of hydrogen bonds between convolutions of the alpha helix. Two key developments in the modeling of the modern α helix were (1) the correct bond geometry, thanks to the crystal structure determinations of amino acids and peptides and pauling's prediction of planar peptide bonds; and (2) the relinquishing of the assumption of an integral number of residues per turn of the helix. the pivotal moment came.

Comments are closed.